Compound containing phosphorous oxy-group and pyridine unit, and method for preparing same
A compound, phosphorus-oxygen technology, applied in the field of organic electroluminescence, the field of preparation of compounds containing phosphorus-oxygen and pyridine units, can solve problems such as insufficient electron transport capacity, achieve improved luminous efficiency, and simple synthesis method Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] An electron transport material with a high triplet energy level, namely 3,3',3'',3'''-(5,5'-(phenylphosphoroxy)bis(benzene-5,3,1) -3)) The preparation method of tetrapyridine, the reaction process is as follows:
[0041]
[0042] The specific reaction steps are:
[0043] 1. Preparation of the initial product 4,4'-(5-bromo-1,3-diphenyl)dipyridine: Weigh 5g 1,3,5-tribromobenzene, 4.88g pyridine-4-boronic acid, 0.73g Pd (PPh 3 ) 4 , 20ml 2M K 2 CO 3 , 20ml ethanol, 80ml toluene were added to a 250ml three-necked flask, heated to 95 ℃, nitrogen protection. After 21 hours of reaction, the reaction was stopped, the reaction solution was poured into water, the organic layer was separated, the aqueous layer was extracted three times with dichloromethane, the organic layers were combined, the organic layer was washed with saturated brine, and the organic layer was dried with anhydrous magnesium sulfate. It was filtered the next morning, and the filtrate was spin-dried with a rotary...
Embodiment 2
[0054] LUMO and HOMO energy level analysis of compound 1
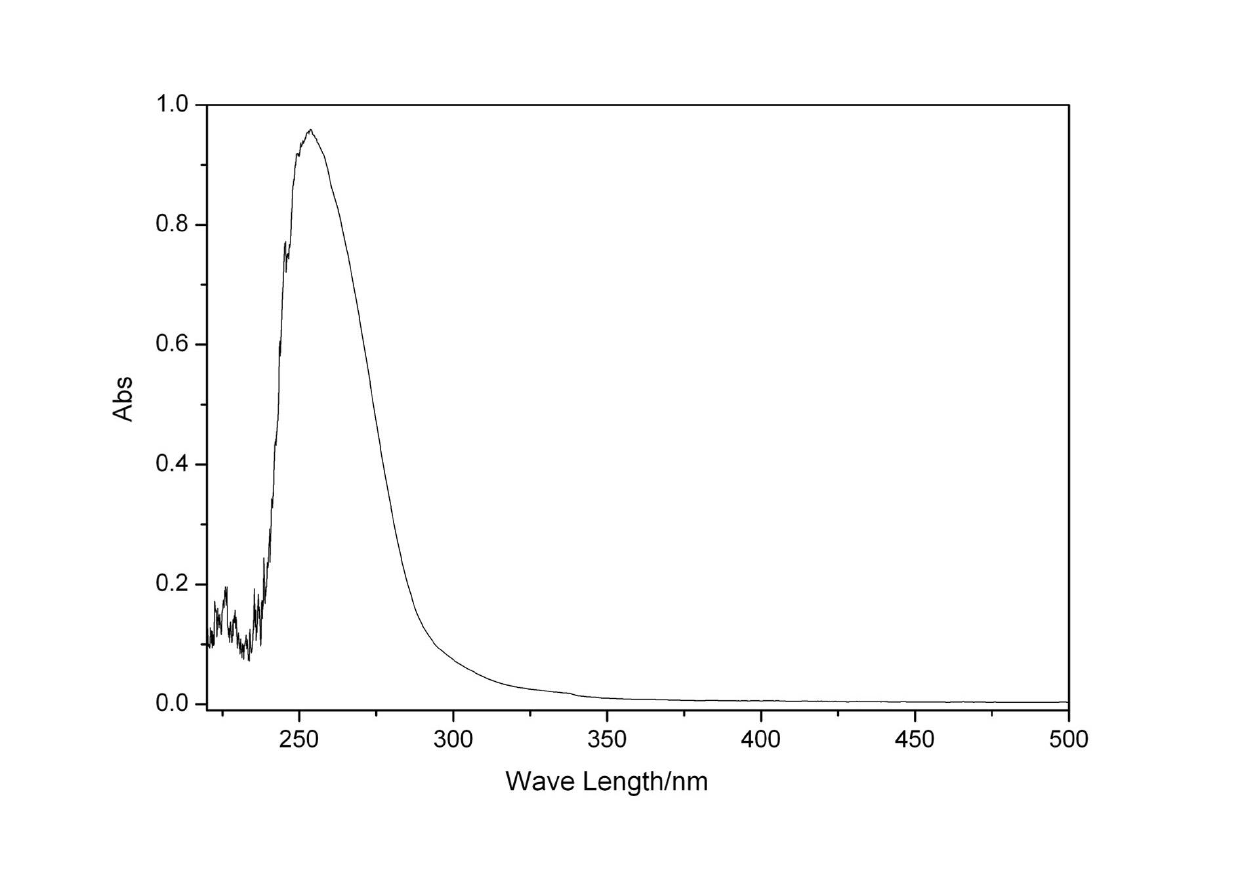
[0055] Perform UV absorption, fluorescence emission and cyclic voltammetry tests on compound 1, from Figure 4 In the cyclic voltammetry spectrum, the HOMO of compound 1 is 6.61eV, and then from image 3 The UV absorption and fluorescence emission spectra show that the LUMO of compound 1 is 2.4 eV, which can prove that compound 1 has better electron affinity and ion potential.
Embodiment 3
[0057] An electron transport material with a high triplet energy level is used in a blue phosphorescent device. The electron transport material is composed of a phosphoroxy group with a high triplet energy level and a pyridine unit with electron transport capability.
[0058] The phosphorescent host material with bipolar carrier transport ability uses ITO as the anode, HAT as the hole injection material, NPB as the hole transport material, mCP as the electron blocking material, and compound 1, That is, the final product prepared in Example 1 of the present invention is an electron transport material, FIrpic is a blue-doped luminescent material, and 2,6-DCzPPY is a host material , LiF is an electron injection material, Al is a cathode, and a device is prepared. Among them,
[0059] Device structure ( Image 6 ) Is: ITO / HAT(20nm) / NPB(30nm) / mcp(5nm) / 2,6-DCzPPY: FIrpic(8wt%,30nm) / compound 1 (20nm) / LiF(1nm) / Al(100nm);
[0060] Table 1: Reported materials and materials of the p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com