High expression and production method of recombinant human thrombin in animal cell
A technology of human thrombin and thrombin, applied in the biological field, can solve the problems of rising production costs and low expression efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Embodiment 1: Preparation of human prothrombin-2 gene
[0071] step one,
[0072] Using the extracted human hepatocyte mRNA as a template and Oligo-dT as a primer, the first-strand cDNA was synthesized by reverse transcription. Using cDNA as template, PCR reaction was carried out with high-fidelity DNA polymerase. Its primer sequence is as follows:
[0073] Primer 1: tgctgcggatccgcaaacgtcgtaccgccaccagtgagta (SEQ ID NO: 11);
[0074] Primer 2: atatgcggccgcctactctccaaactgatcaatga; (SEQ ID NO: 12)
[0075] Primer 3: tgctccagcgggtccggcgaaccgccaccagtgagtacca (SEQ ID NO: 13)
[0076] Primer 1 contains the C-terminal sequence of human protein C signal peptide and the N-terminal coding sequence of human prothrombin-2. Primer 2 contains the C-terminal coding sequence of human prothrombin-2 and introduces a NotⅠ restriction site. The chimeric gene of the peptide partial sequence and the complete coding region of human prothrombin-2, its sequence is as SEQ ID NO:1
[0077] P...
Embodiment 2
[0089] Example 2: Construction of Human Prothrombin-2 Expression Plasmid
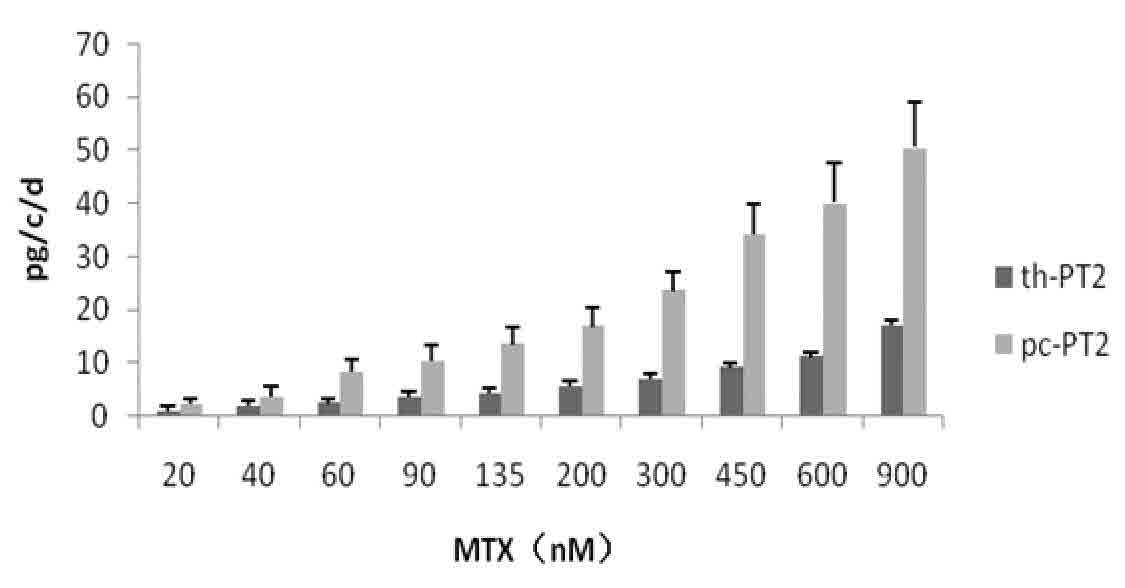
[0090] The amplified product and the pGN vector (see Chinese patent 200910052576.1) were double-digested with KpnI and NotI respectively, and after the digested product was purified, the amplified product: pGN vector (molar ratio 3:1) was ligated with T4 DNA ligase to form ring. Construct the expression plasmids pGN / pc-PT2 and pGN / th-PT2, see the plasmid map image 3 .
Embodiment 3
[0091] Example 3: Using animal cells to express human prothrombin-2
[0092] The expression plasmid described in Example 2 was used to transfect conventional DHFR-deficient Chinese hamster ovary cells CHO DG44 (G Urlaub et al., somatic cells, Mol. Genet., 12, p555-556, 1986, hereinafter referred to as CHO).
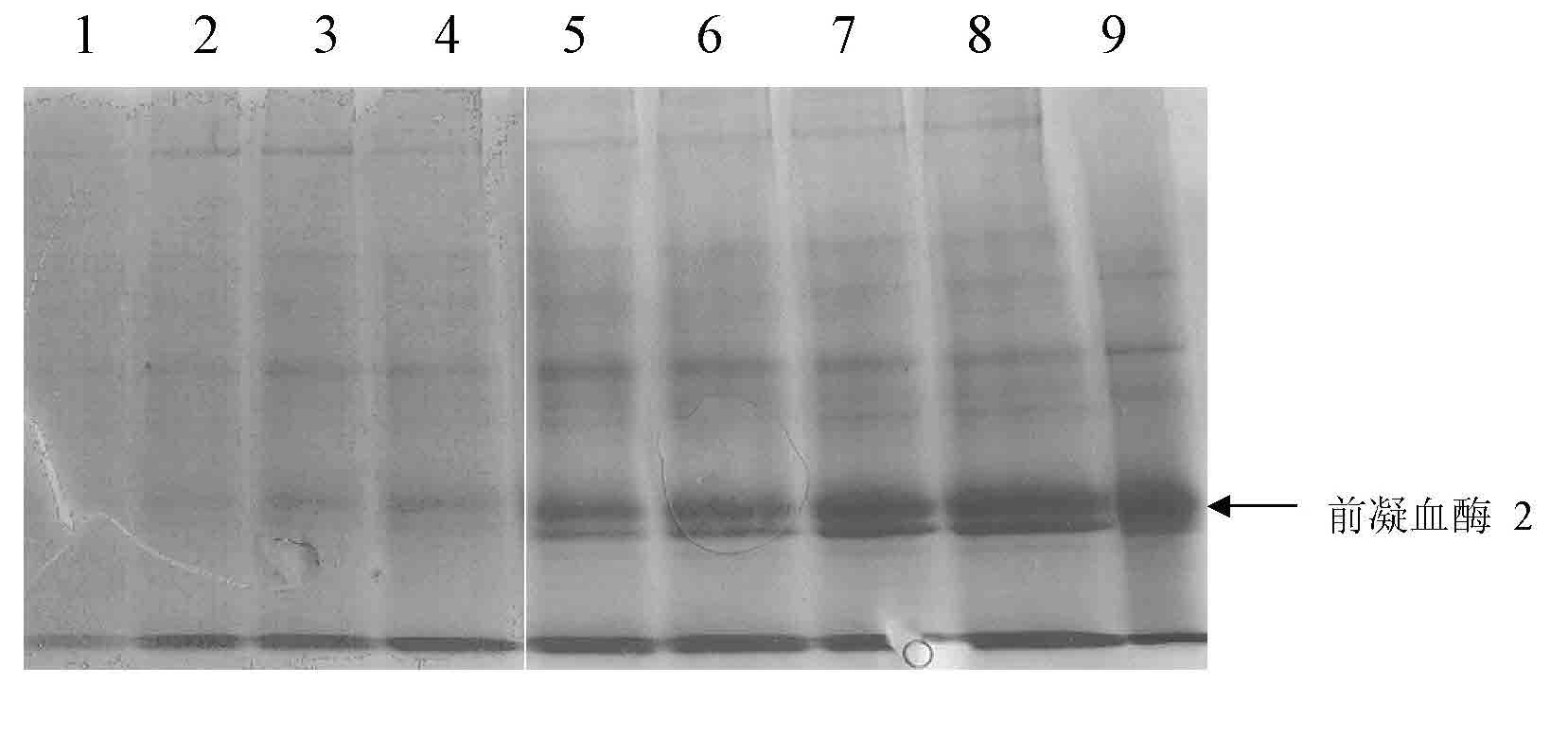
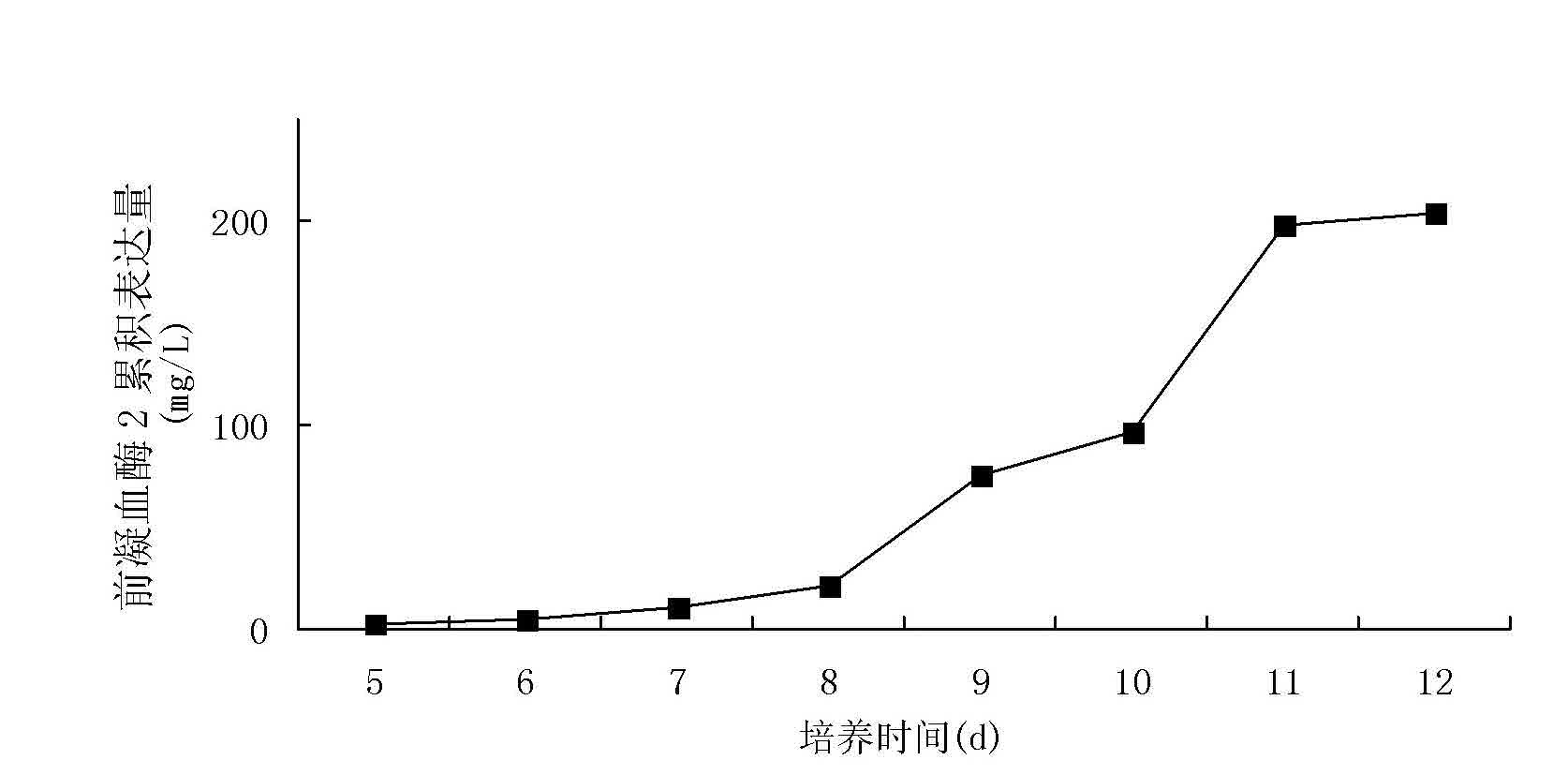
[0093] The cells were cultured normally by adding hypoxanthine and thymine (HT) in commercial serum-free Excell-302 medium (Sigma Company), and the transfection was performed by electroporation. The process was as follows: CHO cells in logarithmic growth phase 24 hours before transfection, centrifuge to change the medium, resuspend with fresh medium and pipette into a single cell suspension; centrifuge the cells and follow the 10 7 Resuspend the cells at the density per milliliter, take 0.8 mL and put it into a 0.4 cm electric shock cup; add 200 micrograms of expression plasmid pGN / pc-PT2 or pGN / th-PT2, mix gently to avoid air bubbles; ice bath for 10 Minutes; electrot...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com