Pharmaceutical composition for treating gout and application thereof
A composition and medicine technology, applied in the field of medicine, can solve the problem of high incidence of gout, and achieve the effects of promoting uric acid excretion and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
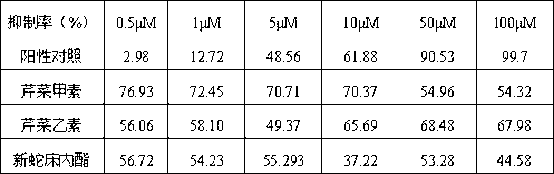
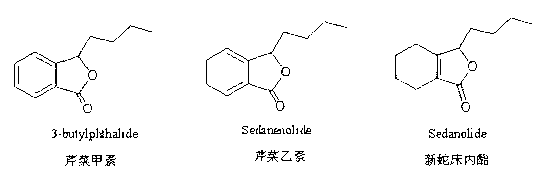
[0025] The preparation of embodiment 1 apigenin A, apigenin B, neocnidolide one
[0026] 1 material
[0027] Celery seed; column chromatography filler: silica gel (100-200 mesh, 200-300 mesh), purchased from Zhifu Silica Gel Development and Experimental Factory in Yantai, Shandong; ethanol, ethyl acetate (EtOAc), petroleum ether (PE), etc. Products of Group Chemical Reagent Co., Ltd. The ethanol used for extraction is industrial grade 95% ethanol, and the rest is analytically pure.
[0028] 2 Methods and results
[0029] Celery seed medicinal material 2 kg was extracted with 8 times the amount of 80% ethanol under reflux for 3 times, each time for 1.5 h. The extract was collected under reduced pressure at 55°C and concentrated to about 400 mL with ethanol, then 400 mL of 95% ethanol was added to the concentrated solution, heated to dissolve, 1 kg of silica gel (100-200 mesh) was added to the solution to mix the sample, and the column was loaded. Pack 1.5 kg of silica gel...
Embodiment 2
[0031] Example 2 Preparation of Apigenin A, Apigenin B and Neocnidolide II
[0032] 1 material
[0033] Celery seed; column chromatography filler: silica gel (100-200 mesh, 200-300 mesh), purchased from Zhifu Silica Gel Development and Experimental Factory in Yantai, Shandong; ethanol, ethyl acetate (EtOAc), petroleum ether (PE), etc. Products of Group Chemical Reagent Co., Ltd. The ethanol used for extraction is industrial grade 95% ethanol, and the rest is analytically pure.
[0034] 2 Methods and results
[0035] Celery seed medicinal material 2 kg was extracted with 8 times the amount of 80% ethanol under heat reflux for 3 times, each time for 1.5 hours. The extract was collected under reduced pressure at 55°C and concentrated to about 400 mL with ethanol, then 400 mL of 95% ethanol was added to the concentrated solution, heated to dissolve, 1 kg of silica gel (100-200 mesh) was added to the solution to mix the sample, and the column was loaded. Pack 1.5 kg of sili...
Embodiment 3
[0037] Embodiment 3 pharmaceutical composition of the present invention one
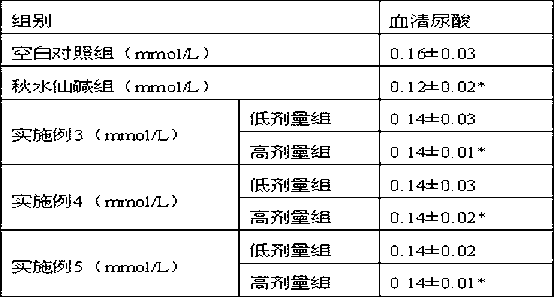
[0038] 30 parts of apigenin A, 19 parts of apigenin B, and 51 parts of neocnidolide are mixed directly.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com