High-dissolving-rate ilepcimide drug composition and preparation method thereof
A technology of composition and medicine, which is applied in the field of eleximide pharmaceutical composition with high dissolution rate and its preparation, can solve problems such as high cost, difficult industrial production, and complexity, and achieve good absorption, improved bioavailability, high dispersion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
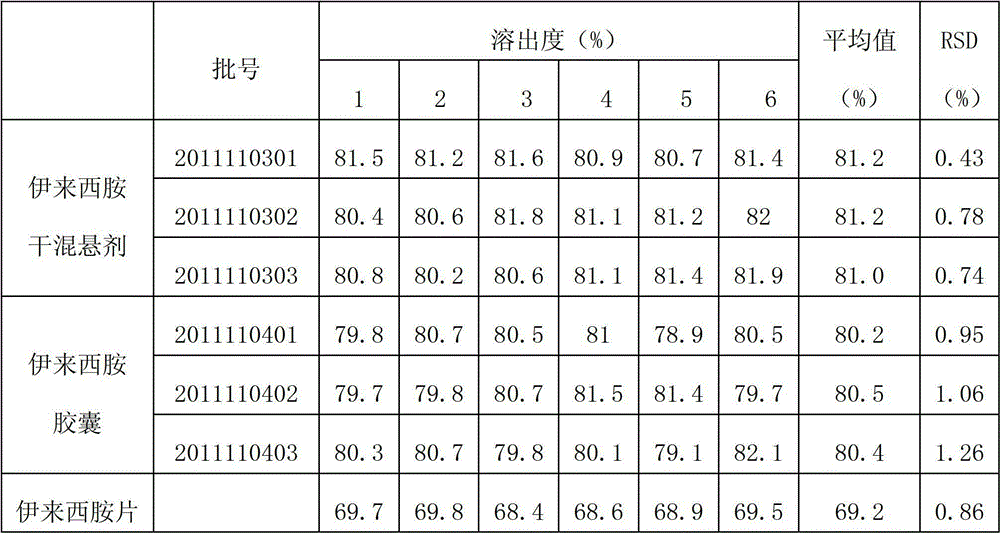
[0056] Embodiment 1: Ileximide Capsules
[0057]
[0058] Preparation Process:
[0059] (1) Pass the raw and auxiliary materials through a 100-mesh sieve for later use;
[0060] (2) Weigh according to the prescription amount, mix eleximine and starch evenly, add ethanol (50%) in an appropriate amount to moisten, and make a soft material;
[0061] (3) Pass the soft material through a 40-mesh sieve to granulate, dry at 45-55°C, granulate with a 40-mesh sieve, then add the prescribed amount of magnesium stearate, and mix well;
[0062] (4) Intermediate detection;
[0063] (5) Capsule filling, polishing, and packaging to obtain Ileximide capsules with a specification of 25 mg (calculated as Ileximide).
Embodiment 2
[0065] Ileximide Capsules Prescription:
[0066]
[0067] Preparation Process:
[0068] (1) Pass the raw and auxiliary materials through a 100-mesh sieve for later use;
[0069] (2) Weigh according to the prescription amount, mix eleciamine and lactose evenly, add ethanol (50%) to moisten it in an appropriate amount, and make a soft material;
[0070] (3) Pass the soft material through a 40-mesh sieve to granulate, dry at 45-55°C, granulate with a 40-mesh sieve, then add the prescribed amount of micro-powder silica gel and mix well;
[0071] (4) Intermediate detection;
[0072] (5) Capsule filling, polishing, and packaging to obtain Ileximide capsules, with a specification of 50 mg (calculated as Ileximide).
Embodiment 3
[0074] Ileximine dry suspension (suspension granules) prescription:
[0075]
[0076] Preparation Process:
[0077] (1) Pass the raw and auxiliary materials through a 100-mesh sieve for later use;
[0078] (2) Weigh according to the prescription amount, mix eleximine, sucrose, mannitol, and xanthan gum evenly, add ethanol (50%) to moisten it in an appropriate amount, and make a soft material;
[0079] (3) Pass the soft material through a 40-mesh sieve to granulate, dry at 45-55°C, granulate with a 40-mesh sieve, then add the prescribed amount of micro-powder silica gel and mix well;
[0080] (4) Intermediate detection;
[0081] (5) Filling and packaging, that is, deleximide dry suspension (suspension granules), with a specification of 50 mg (calculated as ileximide).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com