Two-step enzyme measuring method and measuring reagent for creatinine in blood serum
A determination method and two-step enzyme technology, which are applied in the field of medical testing, can solve the problems of poor accuracy and precision of reagents, poor repeatability of clinical results, and inability to meet the requirements of routine testing.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Reagent 1:
[0038] Reagent 2:
[0039] The preparation method of Reagent 1 and Reagent 2 is a conventional method, that is, the components of Reagent 1 and Reagent 2 are added to distilled water and then mixed and stirred evenly.
Embodiment 2
[0041] Reagent 1:
[0042] Reagent 2:
[0043] The preparation method of embodiment 2 reagent is the same as embodiment 1.
Embodiment 3
[0045] Reagent 1:
[0046] Reagent 2:
[0047]
[0048] The preparation method of embodiment 3 reagent is the same as embodiment 1.
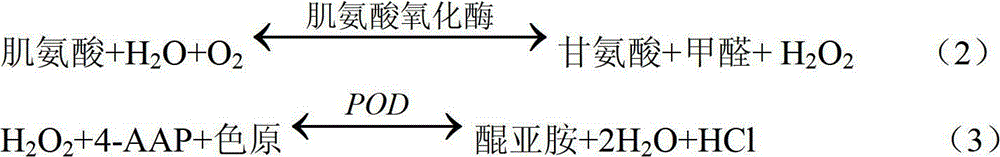
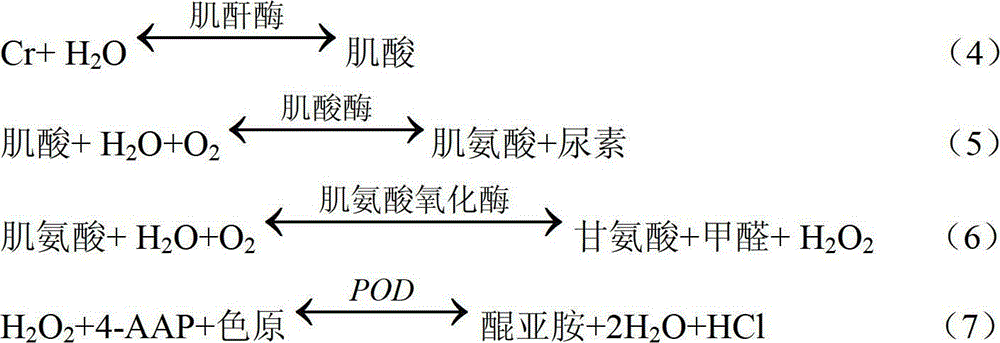
[0049] The test conditions for the determination of Cr in the sample by the reagent of the present invention are as follows: temperature: 37° C.; optical path of the cuvette is 1.0 cm. The main detection wavelength is 546nm, and the secondary wavelength is 700nm.
[0050] The method of measuring Cr in the sample by using the Cr determination reagent of the present invention is as follows: add R1 to the sample (calibration tube as the sample) and mix evenly, add R2 and mix evenly after incubating at 37°C for 5 minutes, record the absorbance A1, and react at 37°C for 5 minutes, Record the absorbance A2. The sample volume is 8 μl, the reagent 1 volume is 200 μl, and the reagent 2 volume is 100 μl.
[0051] The Cr content in the reagent of the present invention measures sample is calculated by the following formula:
[0052]
[0053] Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com