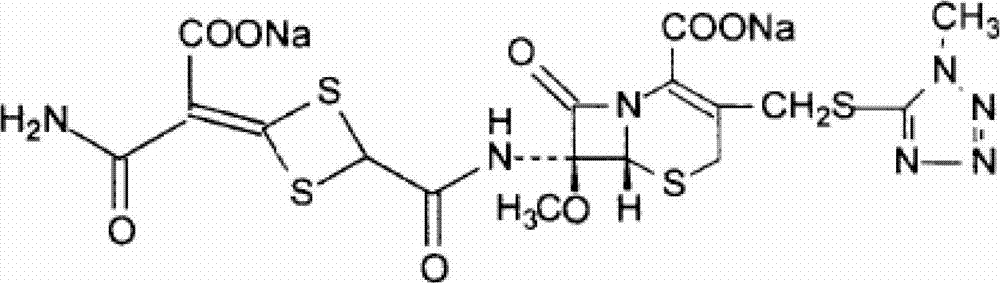
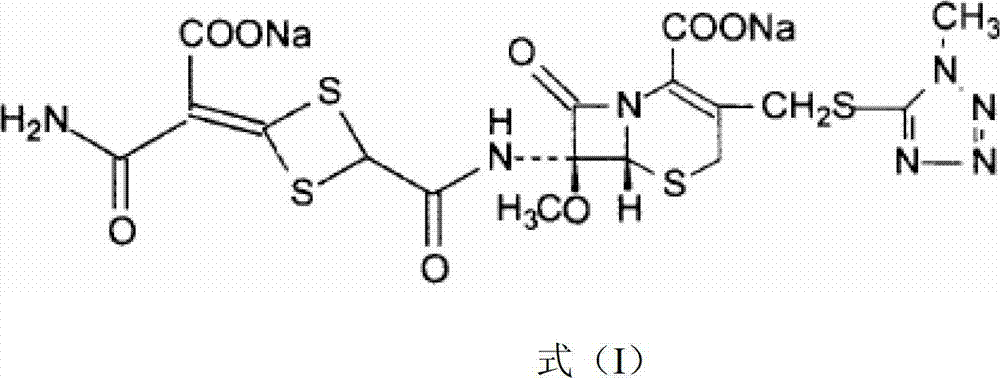
Cefotetan disodium compound as well as preparation method and medicinal composition thereof
A technology of cefotetan disodium and compounds, applied in organic chemistry, antibacterial drugs, pharmaceutical formulations, etc., can solve problems such as difficulties in production, storage and use, instability of cefotetan disodium, and unqualified products, and achieve Stable product quality, avoiding potential safety hazards, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] [Example 1] Preparation of Cefotetan Disodium Compound
[0068] 1) Dissolve cefotetan disodium in a mixed solvent of tetrahydrofuran and methanol to obtain a tetrahydrofuran / methanol solution of cefotetan disodium;
[0069] 2) Add chloroform dropwise to the tetrahydrofuran / methanol solution of cefotetan disodium obtained in step 1) under an ultrasonic field until crystals are precipitated;
[0070] 3) Turn off the ultrasonic field, stand still, filter, wash the filter cake with methanol, and dry to obtain the cefotetan disodium compound.
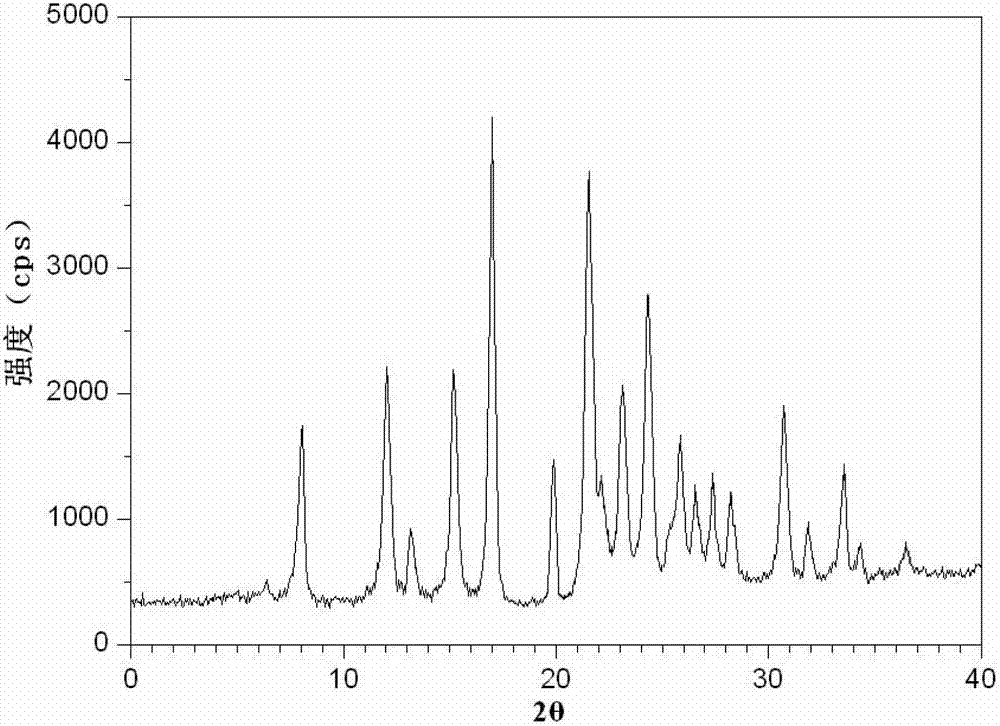
[0071] The characteristic peaks in the X-ray powder diffraction pattern of the obtained cefotetan disodium compound measured using Cu-Kα rays are 8.0°, 12.1°, 15.4°, 17.0°, 19.8°, 21.6°, 23.0°, 2θ. 24.3°, 25.7°, 27.4°, 30.7° and 33.5° display.
[0072] The following are Examples 2-9, the preparation method is the same as that of Example 1, and the specific process parameters are shown in Table 1:
[0073] Table 1
[0074]
[0075] The characteri...
preparation Embodiment 1
[0076] [Formulation Example 1] Cefotetan disodium freeze-dried powder injection
[0077]
[0078] Preparation Process:
[0079] (1) Weigh the prescription amount of the cefotetan disodium compound prepared in Example 1 and add it to 80% of the total volume of water for injection, and stir to obtain a solution;
[0080] (2) Determine the pH of the solution (range from 4.5 to 5.5), adjust the pH with 0.1 mol / L dilute hydrochloric acid if necessary;
[0081] (3) Add water for injection to 100%;
[0082] (4) Add 0.1% activated carbon, stir, let stand for 20 minutes, filter and decarbonize, filter with 0.22μm filter membrane;
[0083] (5) Intermediate testing;
[0084] (6) Fill the above-mentioned solution according to the volume of 4ml / piece (specification: 1g), stopper half, freeze-dry, stopper and cap.
[0085] (7) Fully inspect the above-mentioned freeze-dried products and obtain them after they are qualified.
preparation Embodiment 2
[0086] [Formulation Example 2] Cefotetan disodium freeze-dried powder injection
[0087]
[0088] Preparation process: the same as in Example 1, except that the cefotetan disodium compound used was prepared in Example 2. In step (6), it was filled with 8ml / piece (specification: 2g).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com