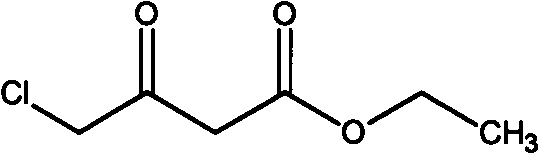
Method for preparing 4-chloroacetoacetic acid ethyl ester
A technology of ethyl chloroacetoacetate and chlorine gas is applied in the chemical field, and can solve the problems of difficult industrialization, complicated process and high cost, and achieve the effects of low cost, simple equipment requirements and safe feeding method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 100g of dichloromethane into a 250ml three-neck flask, add 15g of diketene at one time, under stirring, the temperature of the reaction solution drops to -5°C, feed 13g of chlorine gas, complete in 1 hour, control the reaction temperature at -5~0°C, pass After that, continue stirring at the same temperature for half an hour. Add 8.6g of ethanol dropwise to the reaction solution, and keep the temperature of the reaction solution at 0°C. After dropping, raise the temperature of the reaction solution to 25°C and stir for 1 small test; fractionate the reaction solution, and recover dichloromethane until there is no fraction Flow out, then residual reaction solution is carried out rectification, collects 22g of colorless transparent liquid, is target product ethyl 4-chloroacetoacetate. The yield of ethyl 4-chloroacetoacetate in this example was 75%.
Embodiment 2
[0025] Add 200g of dichloromethane into a 500ml three-necked flask, add 50g of diketene at one time, under stirring, the temperature of the reaction solution drops to -5°C, feed 44g of chlorine gas, and complete in 1 hour, control the reaction temperature at -5~0°C, pass After that, continue stirring at the same temperature for half an hour. Add 28.7g of ethanol dropwise to the reaction solution and keep the temperature of the reaction solution at 0°C. After dropping, raise the temperature of the reaction solution to 25°C and stir for 1 small test; fractionate the reaction solution and recover dichloromethane until there is no fraction Until it flows out, then the remaining reaction solution is rectified, and 78.4 g of a colorless transparent liquid is collected, which is the target product ethyl 4-chloroacetoacetate. The yield of ethyl 4-chloroacetoacetate in this example was 80%.
Embodiment 3
[0027] Add 200g of dichloromethane into a 500ml three-necked flask, add 50g of diketene at one time, and under stirring, the temperature of the reaction solution drops to -30°C, inject 44g of chlorine gas, and complete in 1.5 hours. Control the reaction temperature at -10~-5°C, After passing through, continue to stir at the same temperature for half an hour. Add 28.7g of ethanol dropwise to the reaction solution, and keep the temperature of the reaction solution at 0°C. After dropping, raise the temperature of the reaction solution to 25°C and stir for 1 hour; carry out fractional distillation of the reaction solution, and recover dichloromethane until no fraction flows out Till then, remaining reaction liquid is carried out rectification, collects 83.3g of colorless transparent liquid, is the target product ethyl 4-chloroacetoacetate. The yield of ethyl 4-chloroacetoacetate in this example was 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com