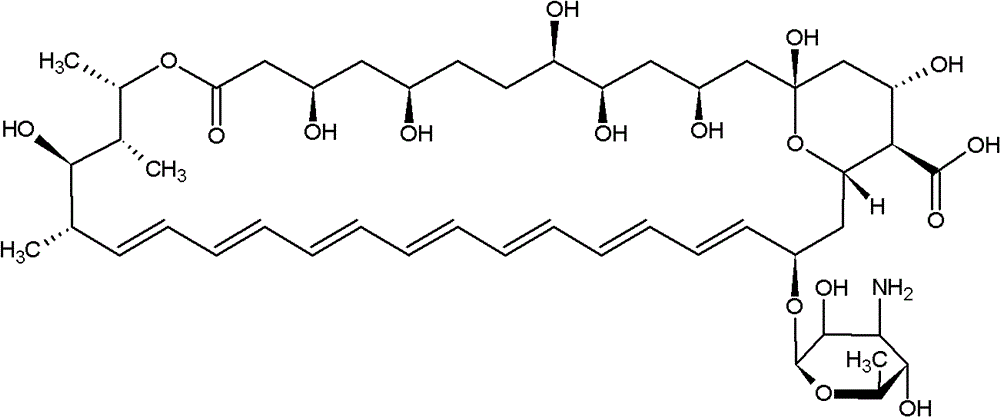
Technology for purifying medicines for treating deep fungal infection
A deep fungus and drug technology, applied in the direction of sugar derivatives, sugar derivatives, organic chemistry, etc., can solve the problems of high cost, complicated process, low yield, etc., and achieve high yield, simple pretreatment, and good adsorption effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Step 1: Put 8.47g (10 billion total) of Amphotericin B in 150ml DMF and 200ml 80% methanol water solvent, adjust the pH to 2.0-4.0 with citric acid to dissolve, and filter the dissolved solution to obtain a clear filtrate;
[0023] Step 2: Pretreat A resin and B resin with DMF and methanol. A resin uses AM-7 resin and B resin uses AM-18 resin. Take 170ml AM-7 resin and 230ml AM-18 resin respectively by wet method Load it into a chromatography column with a column diameter ratio of 12.5:1, pass the filtrate through a mixed resin column, and control the outflow rate to 2-10ml / min; then add the filtrate from the column to a mixture of 100ml dichloromethane and 100ml water for injection In the solvent, adjust the pH to 4.0-6.0 with triethylamine to crystallize; after crystallization, the solution is placed in a water bath and slowly heated to 35-45°C, and kept for 18-24 hours to grow crystals.
[0024] Step 3: The obtained crystal growth solution is suction filtered and sent to ...
Embodiment 2
[0027] Step 1: Put 462.96g (50 billion total) of Amphotericin B into 10LDMF and 10L of 80% methanol water solvent, adjust the pH to 2.0-4.0 with citric acid to dissolve, and filter the dissolved solution to obtain a clear filtrate;
[0028] Step 2: Pretreat macroporous resins of HPD-100 and HPD-500 with DMF and methanol, respectively, weigh 11.4L of HPD-100 and 13.6L of HPD-500 resin into the column diameter ratio of 12.5 by wet method :1 chromatographic column, pass the filtrate obtained in step 1 through a mixed resin column, and control the outflow rate to 2-10ml / min; then add the filtrate obtained through the column to a mixed solvent of 10L dichloromethane and 12.5L water for injection , Adjust the pH to 4.0-6.0 with citric acid, and crystallize; after crystallization, keep the crystal growing tank at 35-45°C and let it stand for 18-24h.
[0029] Step 3: The crystal growth solution obtained in step 2 is suction filtered and sent to an oven for drying at a temperature of 40° C....
Embodiment 3
[0032] Step 1: Put 847.46g (100 billion total) of Amphotericin B in 18LDMF and 20L methanol (80%) solvent, adjust the pH to 2.0-4.0 with citric acid to dissolve, and filter the dissolved solution to obtain a clear filtrate ;
[0033] Step 2: Pretreat macroporous resins of HPD-100 and HPD-500 with DMF and methanol, respectively, weigh 17.8L of HPD-100 and 22.2L of HPD-500 resin into the column diameter ratio of 12.5. :1 chromatographic column, pass the filtrate obtained in step 1 through a mixed resin column, and control the outflow rate to 2-10ml / min; then add the filtrate obtained through the column to a mixed solvent of 15L dichloromethane and 20L water for injection, Adjust the pH to 4.0-6.0 with citric acid to crystallize; after crystallization, keep the crystal growing tank at 35-45°C and leave it to stand for 18-24 hours.
[0034] Step 3: Filter the obtained crystal growth solution, send the crystal to an oven for drying, at a temperature of 50° C., a pressure of 0.8-1.0 Mpa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com