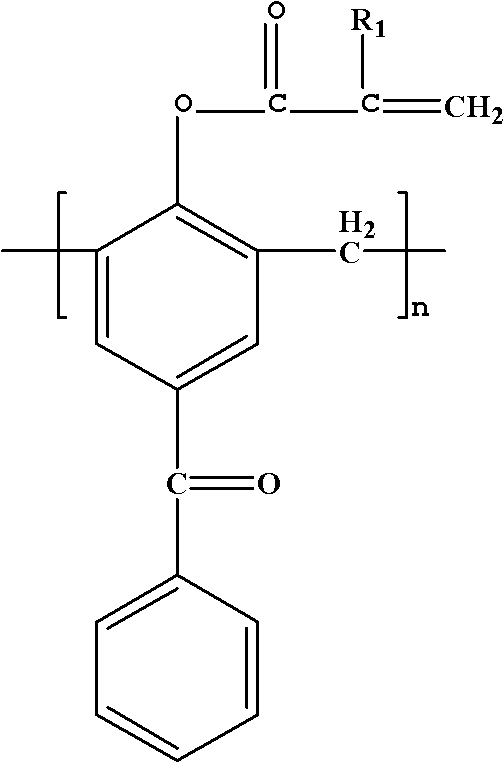
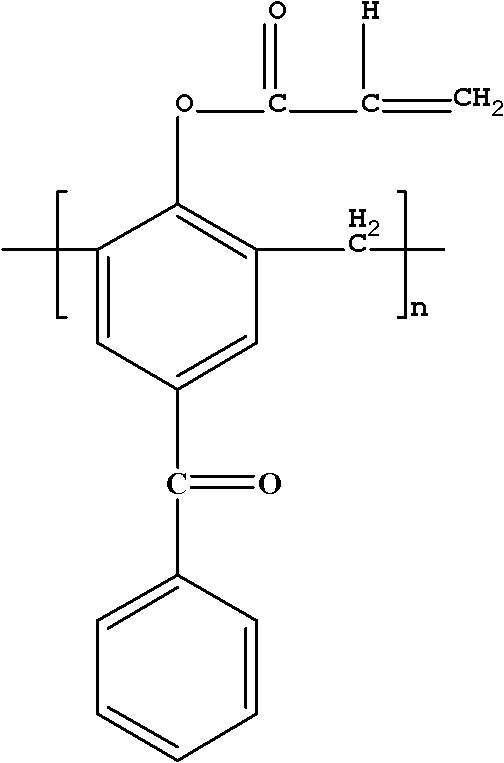
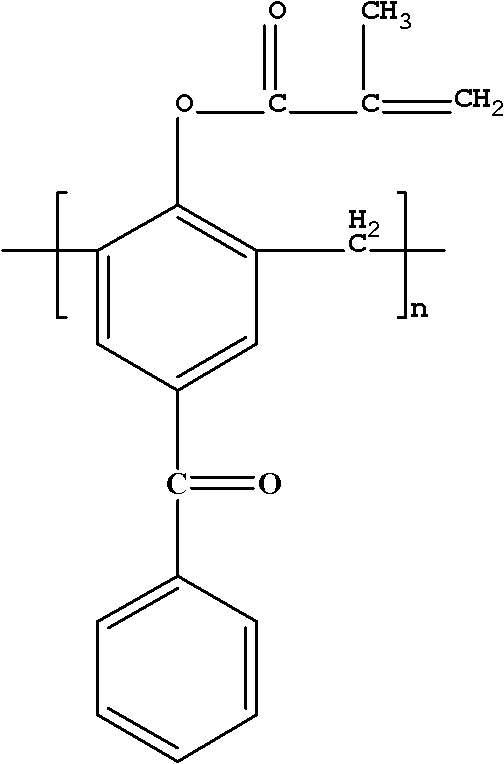
Polymerizable macromolecule photoinitiator and preparation method thereof
A photoinitiator and macromolecule technology, applied in the field of macromolecular photoinitiator preparation, can solve the problems of small molecular weight of the initiator, poor initiation effect, low ultraviolet absorption, etc. The effect of simple operating conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Synthesis of Hydrogen-Substituted Polymerizable Macromolecular Photoinitiators
[0026] Add 10g of 4-hydroxybenzophenone and 4.5g of formaldehyde solution into a three-necked flask, add 0.44g of NaOH, heat and stir to 95°C, and react for 2h; then raise the temperature to 150°C, carry out vacuum filtration reaction for 20min, cool down after the reaction Collect the product at 105°C, pour the product into water to precipitate, and obtain a light yellow macromolecular photoinitiator containing benzophenone after suction filtration. Dissolve the obtained light yellow macromolecular photoinitiator containing benzophenone in 21.2g of dichloromethane, and add 10.21g of triethylamine into the three-necked flask simultaneously for stirring, and dissolve 5.48g of acryloyl chloride in 11g of dichloromethane Slowly add the mixed solution to the three-neck flask dropwise within 3 hours, the reaction temperature is 0-5°C, after the dropwise addition, stir at room temperature for 3 h...
Embodiment 2
[0030] Synthesis of Methyl Substituted Polymerizable Macromolecular Photoinitiators
[0031] Add 10g of 4-hydroxybenzophenone and 4.5g of formaldehyde solution into a three-necked flask, add 0.44g of NaOH, heat and stir to 95°C, and react for 2h; then raise the temperature to 150°C, carry out vacuum filtration reaction for 20min, cool down after the reaction Collect the product at 105°C, pour the product into water to precipitate, and obtain a light yellow macromolecular photoinitiator containing benzophenone after suction filtration. The obtained light yellow macromolecular photoinitiator containing benzophenone was dissolved in 22g of dichloromethane, and 10.21g of triethylamine was added into a three-necked flask simultaneously for stirring, and 6.33g of methacryloyl chloride was dissolved in 13g of dichloromethane In methane, slowly add the mixed solution to the three-neck flask dropwise within 3 hours, the reaction temperature is 0-5°C, after the dropwise addition, stir a...
Embodiment 3
[0035] Synthesis of Methoxyl Substituted Polymerizable Macromolecular Photoinitiators
[0036] Add 10g of 4-hydroxybenzophenone and 4.6g of formaldehyde solution into a three-necked flask, add 0.5g of alkali, heat and stir to 95°C, and react for 2h; Cool to 105°C to collect the product, pour the product into water to precipitate, and obtain a light yellow macromolecular photoinitiator containing benzophenone after suction filtration. The obtained light yellow macromolecular photoinitiator containing benzophenone is dissolved in 21g of dichloromethane, joins in the there-necked flask simultaneously with 10.3g of triethylamine and stirs, and 7.3g of methoxyacryloyl chloride is dissolved in 15g of dichloromethane In methyl chloride, slowly add the mixed solution to the three-necked flask dropwise within 3 hours, the reaction temperature is 0-5°C, after the dropwise addition, stir at room temperature for 3 hours, and let it stand overnight. The yellow liquid obtained above was su...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com