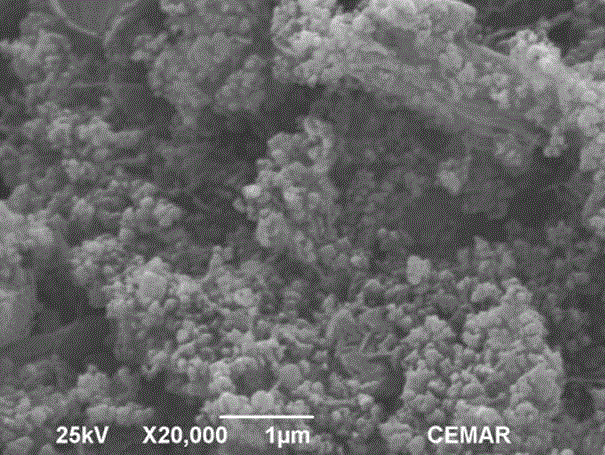
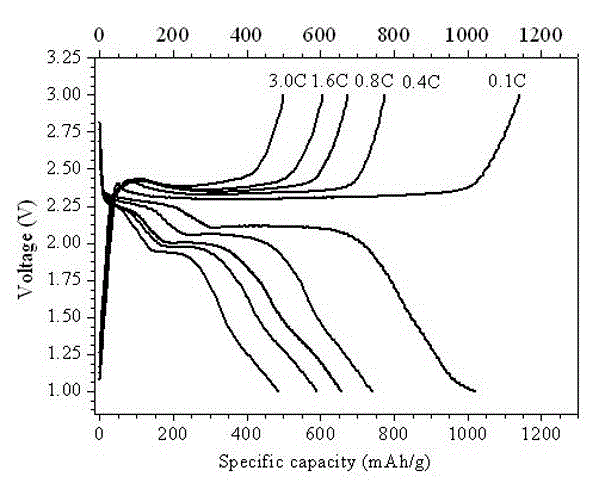
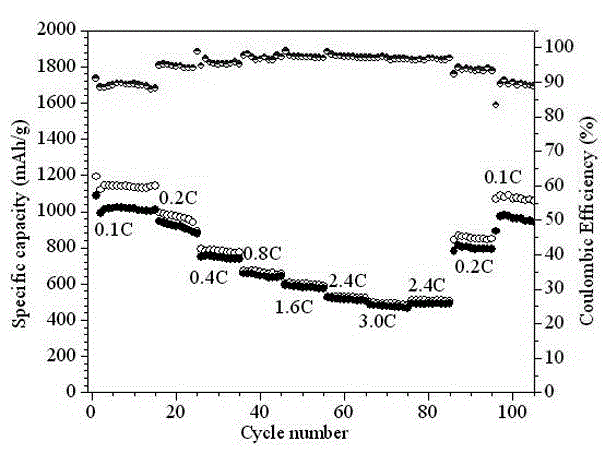
Preparation method of novel porous skeleton MIL-101(Cr)@S/graphene composite material for cathode of lithium sulfur battery
A composite material and graphene technology, applied in battery electrodes, circuits, electrical components, etc., can solve problems such as poor cycle performance and uneven dispersion of coated sulfur, improve adhesion, simple and easy-to-operate preparation method, and promote cycle performance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) Synthesis of MIL-101(Cr) metal-organic framework: 2.0 g of Cr(NO 3 ) 3 9H 2 O and 0.833 g of terephthalic acid H 2Dissolve BDC in 30 mL of deionized water, then add 0.26 mL of HF, move the solution into a 50 mL polytetrafluoroethylene reaction tank after fully dissolving, then place the reaction tank in a stainless steel jacket and seal it; put the reaction tank in The hydrothermal reaction was carried out in a temperature-programmed furnace, and the heating program was as follows: the solution was heated from room temperature to 210 oC at 5 oC / min, and kept for 8 h, and then the solution was lowered to room temperature at a cooling rate of 0.4 oC / min; After the solution was cooled to room temperature, the crystals were washed with water, dimethylformamide (DMF) and ethanol in sequence, centrifuged, filtered and dried to obtain the MIL-101(Cr) metal-organic framework material. (2) Preparation of metal-organic framework MIL-101(Cr)S composite material: mix sulfur ...
Embodiment 2
[0037] (1) Synthesis of MIL-101(Cr) metal-organic framework: 2.2 g of Cr(NO 3 ) 3 9H 2 O and 0.900 g of terephthalic acid H 2 Dissolve BDC in 40 mL of deionized water, then add 0.28 mL of HF, and after fully dissolved, move the solution into a 100 mL capacity polytetrafluoroethylene reaction tank, then place the reaction tank in a stainless steel jacket and seal it; put the reaction tank in The hydrothermal reaction was carried out in a temperature-programmed furnace, and the heating program was as follows: the solution was heated from room temperature to 220 oC at 8.0 oC / min, and kept for 9 h, and then the solution was lowered to room temperature at a cooling rate of 0.5 oC / min; After the solution was cooled to room temperature, the crystals were washed with water, dimethylformamide (DMF) and ethanol in sequence, centrifuged, filtered and dried to obtain the MIL-101(Cr) metal-organic framework material. (2) Preparation of metal-organic framework MIL-101(Cr)S composite mate...
Embodiment 3
[0039] (1) Synthesis of MIL-101(Cr) metal-organic framework: 2.4 g of Cr(NO 3 ) 3 9H 2 O and 0.996 g of terephthalic acid H 2 Dissolve BDC in 50 mL of deionized water, then add 0.30 mL of HF, and after fully dissolved, move the solution into a 100 mL capacity polytetrafluoroethylene reaction tank, then place the reaction tank in a stainless steel jacket and seal it; put the reaction tank in The hydrothermal reaction was carried out in a temperature-programmed furnace, and the heating program was as follows: the solution was heated from room temperature to 220 oC at 10.0 oC / min, and kept for 10 h, and then the solution was lowered to room temperature at a cooling rate of 0.6 oC / min; After the solution was cooled to room temperature, the crystals were washed with water, dimethylformamide (DMF) and ethanol in sequence, centrifuged, filtered and dried to obtain the MIL-101(Cr) metal-organic framework material. (2) Preparation of metal-organic framework MIL-101(Cr)S composite ma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com