Gentamycin sulfate injection containing buffer agent
A technology of gentamicin sulfate and injection, which is applied in the direction of medical preparations of non-active ingredients, inorganic non-active ingredients, active ingredients of heterocyclic compounds, etc., and can solve the problem of increasing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
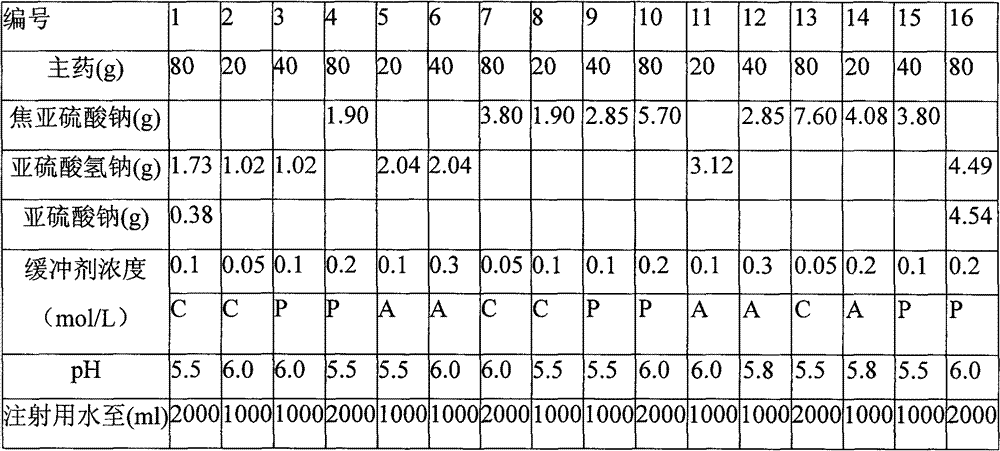
[0034] Preparation Process
[0035] 1) Prepare the prescription amount of pH buffer, add the prescription amount of sodium sulfite and sodium bisulfite and stir until completely dissolved, make up the water for injection to 90% of the prescription volume, and use it for later use;
[0036] 2) Add the prescribed amount of gentamycin sulfate to the standby solution prepared in 1), stir until it is completely dissolved, add 1 g of medicinal charcoal to the standby solution prepared in 2), stir well and let stand for 10 minutes;
[0037] 3) Filter and make up the water for injection to the prescription amount;
[0038] 4) After fine filtration until the visible foreign matter is qualified, potting;
[0039] 5) High temperature sterilization, ready.
Embodiment 2
[0041] Preparation Process
[0042] 1) Prepare the prescription amount of pH buffer, add the prescription amount of sodium bisulfite and stir until it is completely dissolved, make up the water for injection to 90% of the prescription volume, and set aside;
[0043] 2) Add the prescription amount of gentamicin sulfate to the standby solution prepared in 1), and set aside;
[0044] 3) Add 3g of medicinal charcoal to the ready-to-use solution prepared in 2), stir it evenly and leave it for 10 minutes;
[0045] 4) Filter to make up the water for injection to the prescription amount;
[0046] 5) After fine filtration until the visible foreign matter is qualified, potting;
[0047] 6) High temperature sterilization, ready.
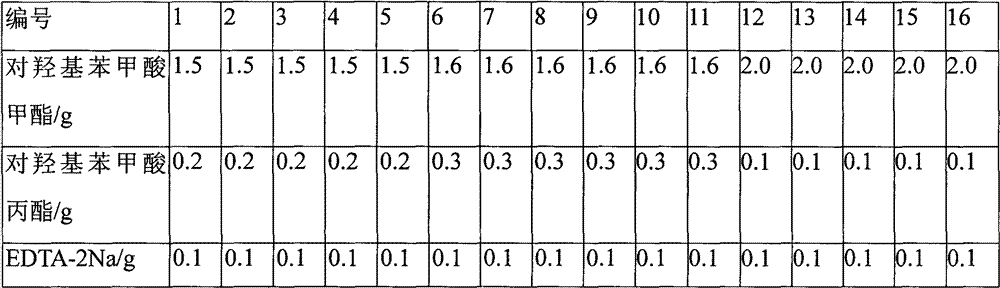
[0048] The preparation process of Example 3-15 is the same as that of Example 2, wherein in Examples 4, 7-10, and 12-15, sodium sulfite is replaced with sodium metabisulfite;
Embodiment 16
[0049] The preparation process of Example 16 is the same as that of Example 1
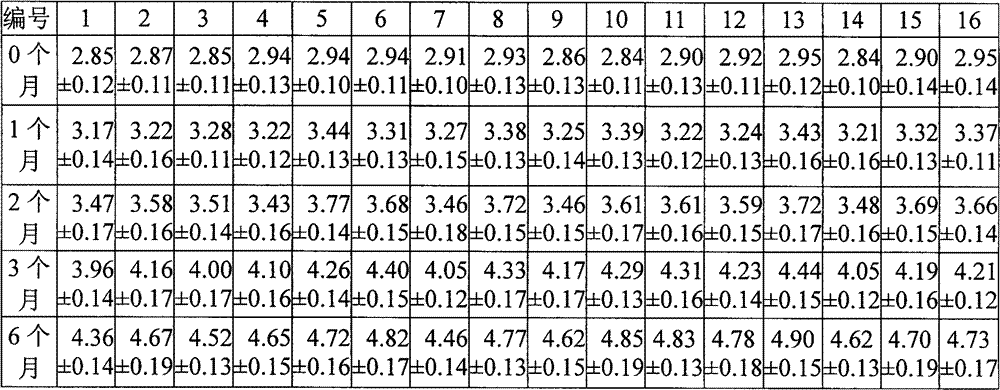
[0050] The formula table of the control example, other prescriptions are the same as the corresponding numbered example, no pH buffering agent is added, sodium carbonate is used as the pH adjuster, adjusted to the same pH value of the corresponding numbered example, and the prescription amount is also calculated according to the amount of 1000 bottles
[0051]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com