Primers for diagnosing ankylosing spondylitis, and method for diagnosing ankylosing spondylitis using the same
An ankylosing spondylitis and primer set technology, applied in the field of primers for the diagnosis of ankylosing spondylitis, can solve the problems of huge exploration and discovery work
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
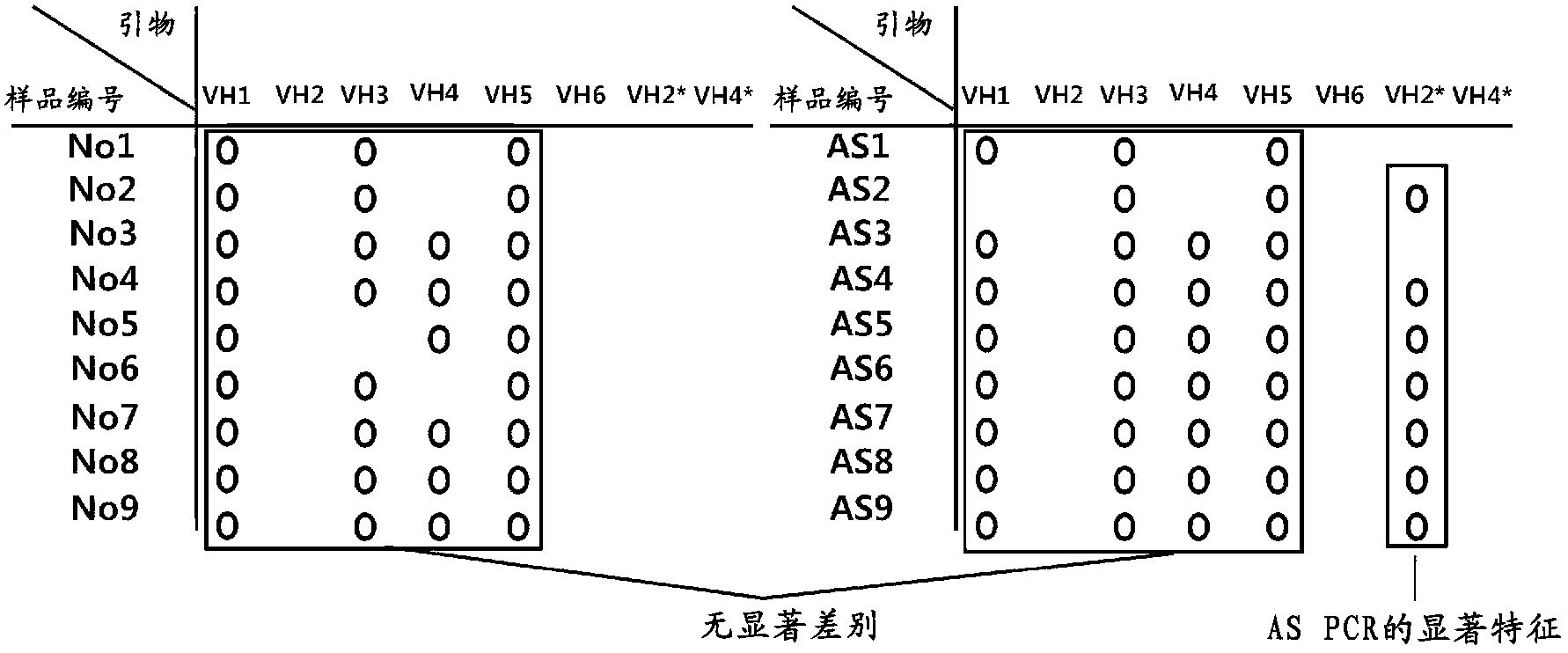
[0062] Example 1. Extension of the VH gene
[0063] After collecting blood from AS patients and normal controls, PBMC (peripheral blood mononuclear cells) and serum were extracted by density gradient centrifugation using a separation medium (Histopaque-sigma, UK) and collected. Perform cDNA synthesis. Subsequently, the VH (variable region of immunoglobulin heavy chain) in each sample was extended from the synthesized cDNA using the VH forward primer and the JH (immunoglobulin heavy chain joining region) reverse primer. Primers are designed based on commonly used primers so that the human VH gene is extended from cDNA (Fan et al., (2003) Clinical Trial Immunology, 131 (2): 364-376 (Van et al., (2003) Clin. Exp.Immunol.131(2):364-376)), by comparing with Immunoglobin Blast HumanVH germline gene sequence (Immunoglobin Blast HumanVH germline gene sequence), adding 3 kinds of primers (with SEQ ID NO: 6 , primers of SEQ ID NO: 7 and SEQ ID NO: 13).
[0064] Table 1
[0065] [s...
Embodiment 2
[0071] Example 2: Transformation of VH2* into bacteria
[0072] The VH2* and PIT2 phagemid vectors obtained in Example 1 were mixed with 4 μl of restriction enzyme NcoI in 100 μl of the total reaction mixture. After incubation at 37°C for 4 hours and purification, 4 μl of restriction enzyme XhoI was added and incubated at 37°C for 4 hours, followed by agarose gel electrophoresis and DNA was isolated by thermal extraction technique.
[0073] Treat the PIT2 phage vector with NcoI and XcoI, add 2 μl of phosphatase, incubate at 37° C. for 1 hour, and then purify. 3 μl of restriction enzyme-cleaved VH2* fragment, and 4 μl of restriction enzyme- and phosphatase-treated PIT2 phagemid vector were added to 20 μl of the total reaction mixture, and 1 μl of ligase was mixed with them , and then the reaction mixture was incubated at 16°C for 18 hours. The incubated reaction mixture was purified and inserted into E. coli TG1 strain by electroporation.
[0074] The reaction conditions o...
Embodiment 3
[0075] Example 3: Sequence Analysis of Transformed Bacterial Strains
[0076] The transformed bacterial strain containing the recombinant DNA obtained in Example 2 was grown on an agar plate containing ampicillin and screened. After screening the transformed strains and culturing them in LB medium for 16 hours, the recombinant DNA in the transformed strains was purified and sequenced with LBM3 primers (with SEQ ID NO: 14; 5'-CAG GAA ACA GCT ATG AC- 3' to identify VH2* fragments in recombinant DNA).
[0077] Insertion of the VH2* fragment into the recombinant DNA was confirmed by DNA sequencing of clones screened by colony polymerase chain reaction. Colony PCR was performed under the same conditions using the primer set for VH2* extension in Example 1 above, and colonies grown on ampicillin-containing media were screened and used as templates DNA.
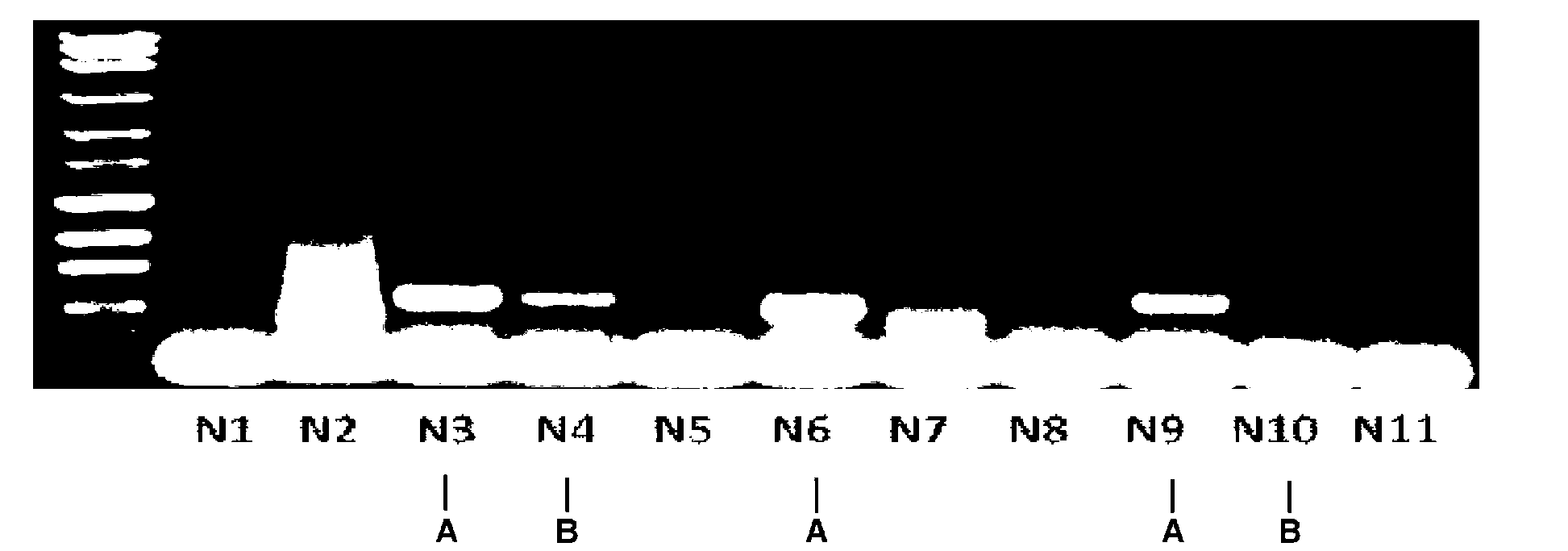
[0078] After electrophoresis of the PCR products, DNA clones with the predicted size were screened. Such as figure 2 As sh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com