Preparation method of hexafluoroethane
A technology of hexafluoroethane and pentafluoroethane, which is applied in the field of preparation of hexafluoroethane, can solve the problems of immature production technology, low output, high energy consumption, etc., and achieve the relief of market demand pressure, long catalyst life, and reaction The effect of low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
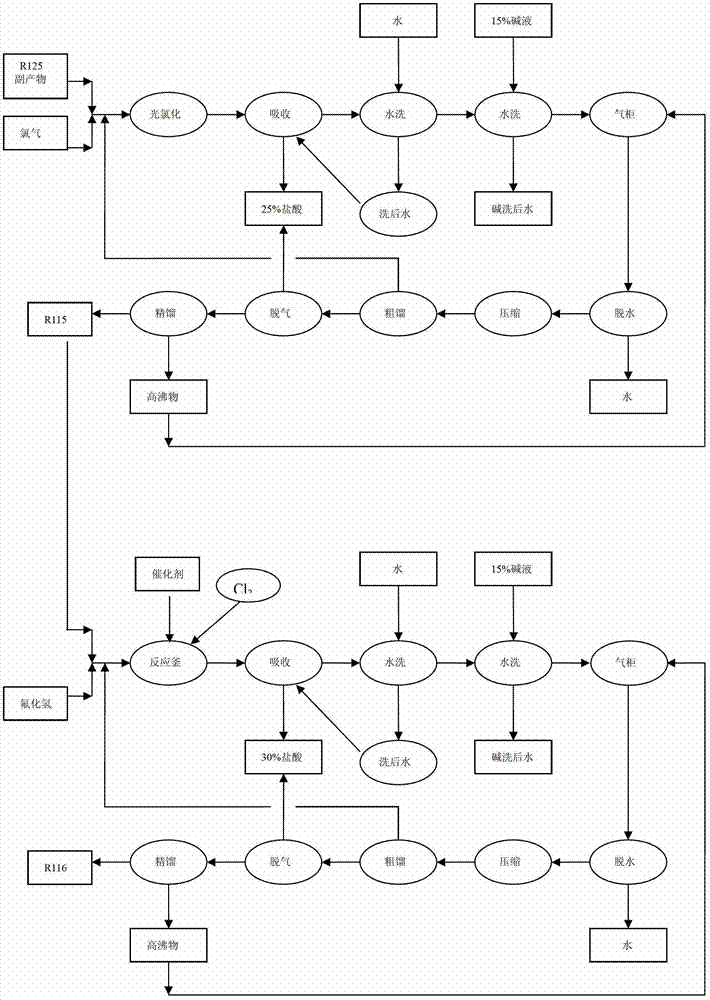
[0021] The preparation method of hexafluoroethane:
[0022] (1) The pentafluoroethane by-product and the chlorine gas mixture are contacted and reacted in the photochemical tower;
[0023] (2) The product chloropentafluoroethane produced in step (1) and the mixed liquid of hydrogen fluoride are contacted and reacted in a reactor equipped with a catalyst, and the catalyst is antimony fluoride.
[0024] The by-product of pentafluoroethane in step (1) is pentafluorochloroethane with a mass fraction of 50% and pentafluoroethane with a mass fraction of 50%.
[0025] The reaction temperature in step (1) is 50°C, and the reaction pressure is 0.1Mpa.
[0026] The molar ratio of pentafluoroethane to chlorine in step (1) is 1:1.3, and the contact time is 30 seconds.
[0027] The reaction temperature in step (2) is 120°C, and the reaction pressure is 2.0Mpa.
[0028] The molar ratio of chloropentafluoroethane to hydrogen fluoride in step (2) is 1:1, and the contact time is 10 seconds....
Embodiment 2
[0033] The preparation method of hexafluoroethane:
[0034] (1) The pentafluoroethane by-product and the chlorine gas mixture are contacted and reacted in the photochemical tower;
[0035] (2) The product chloropentafluoroethane produced in step (1) and the mixed liquid of hydrogen fluoride are contacted and reacted in a reactor equipped with a catalyst, and the catalyst is antimony fluoride.
[0036] The by-product of pentafluoroethane in step (1) is pentafluorochloroethane with a mass fraction of 80% and pentafluoroethane with a mass fraction of 20%.
[0037] The reaction temperature in step (1) is 150°C, and the reaction pressure is 0Mpa.
[0038] The molar ratio of pentafluoroethane to chlorine in step (1) is 1:1.8, and the contact time is 300 seconds.
[0039] The reaction temperature in step (2) is 50°C, and the reaction pressure is 3.0Mpa.
[0040] The molar ratio of chloropentafluoroethane to hydrogen fluoride in step (2) is 1:2, and the contact time is 30 seconds. ...
Embodiment 3
[0045] The preparation method of hexafluoroethane:
[0046] (1) The pentafluoroethane by-product and the chlorine gas mixture are contacted and reacted in the photochemical tower;
[0047] (2) The product chloropentafluoroethane produced in step (1) and the mixed liquid of hydrogen fluoride are contacted and reacted in a reactor equipped with a catalyst, and the catalyst is antimony fluoride.
[0048] The by-product of pentafluoroethane in step (1) is pentafluorochloroethane with a mass fraction of 60% and pentafluoroethane with a mass fraction of 40%.
[0049] The reaction temperature in step (1) is 100°C, and the reaction pressure is 0.05Mpa.
[0050] The molar ratio of pentafluoroethane to chlorine in step (1) is 1:1.5, and the contact time is 100 seconds.
[0051] The reaction temperature in step (2) is 80°C, and the reaction pressure is 2.5Mpa.
[0052] The molar ratio of chloropentafluoroethane to hydrogen fluoride in step (2) is 1:1.2, and the contact time is 20 seco...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com