Easy-disintegrating polymeric micelle composition
A polymer and composition technology, which can be applied in the directions of non-active components of polymer compounds, drug delivery, and medical preparations of non-active components, etc., can solve problems such as hindering the proper release of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
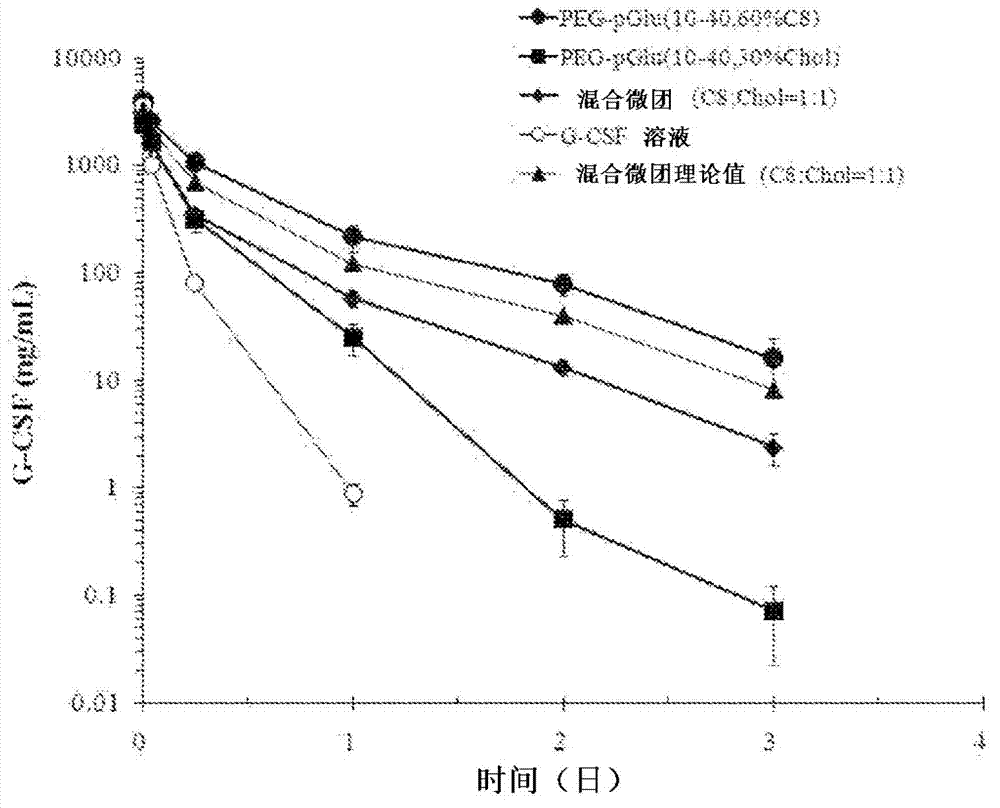
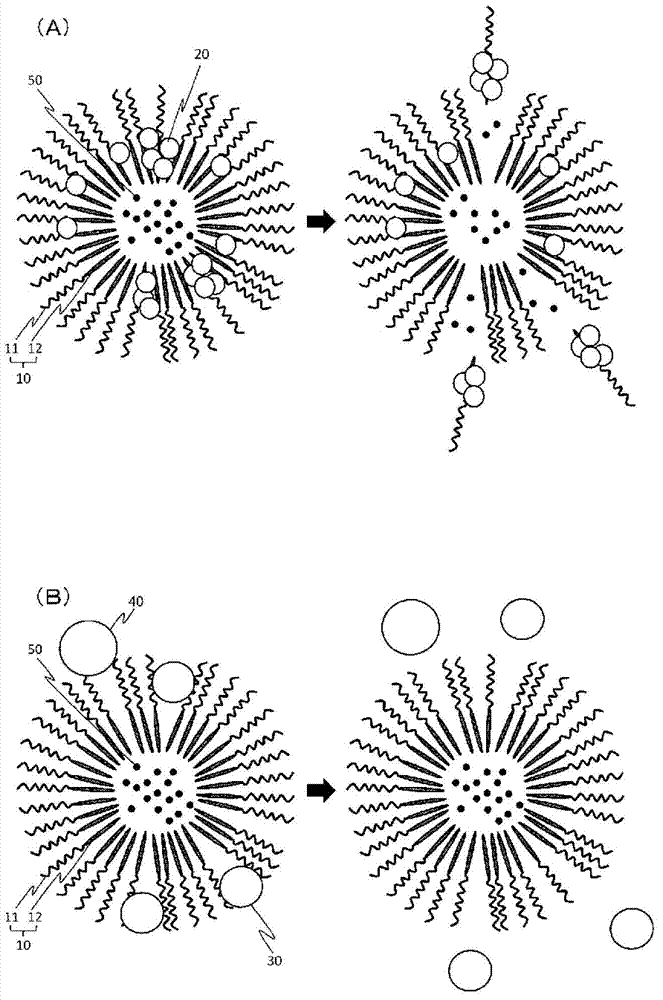
[0090] In the following description, in order to simplify the expression, for example, the hydrophilic polymer segment of the block copolymer includes a PEG chain with an average molecular weight of 10,000, and the hydrophobic polymer segment includes a polyamino acid chain with an average of 40 residues, The benzyl group introduction rate on the side chain of the polyamino acid chain is about 65%, and in this case, it is labeled after the block copolymer (10-40, 65% Bn). Similarly, when the hydrophobic group introduced into the side chain of the polyamino acid chain is an octyl group or a cholesterol group, after the block copolymer, mark (10-40, 65%C8) or (10-40, 65%Chol ). The introduction rate of so-called hydrophobic groups is about 65%, including 62 to 68%.
reference example 1
[0091] [Reference Example 1] Preparation of lysozyme-encapsulated polymer micelles
[0092] As the block copolymer, a block copolymer described in the following general formula (V) and Table 1 was used. Each block copolymer was weighed in a vial, and purified water was added so that the polymer concentration was 5 mg / mL. Next, the polymer solution was vigorously stirred overnight at 4°C. This polymer solution was irradiated with ultrasonic waves (in ice bath cooling, Low, 1 second interval, 10 minutes) using a biolysis apparatus (manufactured by Nippon Seiki Seisakusho, High Power Unit), and then subjected to 0.22 μm membrane filter treatment. Thus, an empty micellar solution with a polymer concentration of 5 mg / mL was obtained. To each empty micelle solution (0.6 mL), 1 mg / mL lysozyme solution (0.15 mL), 200 mM sodium phosphate buffer (pH 6), and purified water were adjusted with 0.1N HCl so that the polymer concentration of 3 mg / mL, the concentration of lysozyme of 0.15 m...
reference example 2
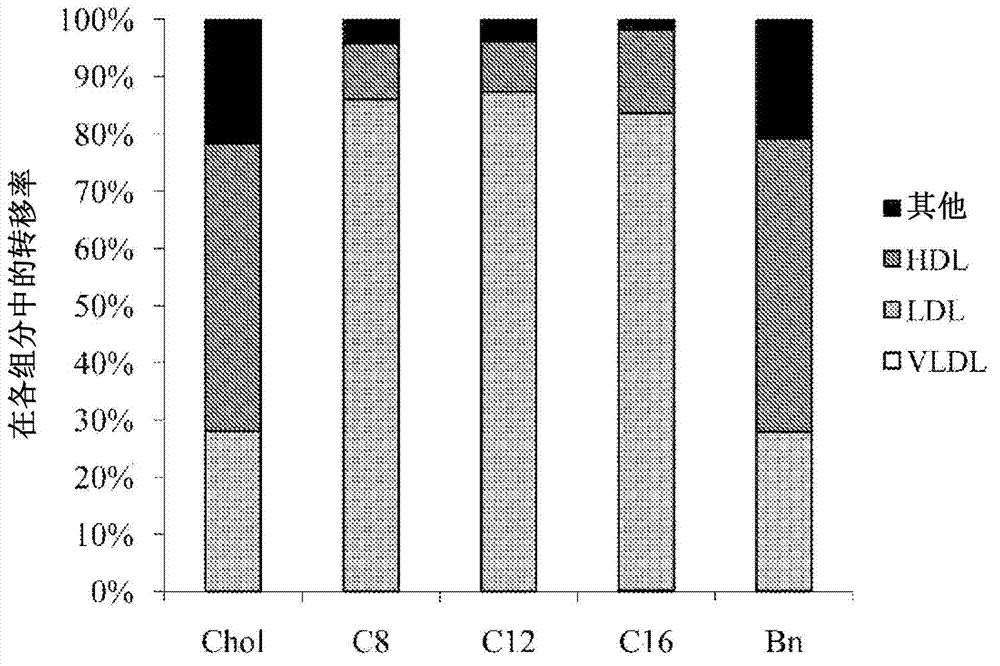
[0097] [Reference example 2] HDL transfer rate
[0098] Using the lysozyme-encapsulated polymer micelles prepared in Reference Example 1, the HDL transfer rate of each block copolymer was determined. The specific experimental method is as follows. Heparinized blood was collected from male Wistar rats at the age of 8 weeks. After centrifugation, 810 μL of rat plasma stored at -80°C was added to 90 μL of lysozyme-encapsulated polymer micelles, and incubated at 37°C for 24 hours (final concentration of lysozyme: 15 µg / mL, polymer final concentration: 300 µg / mL). Next, use the trade name "OptimaMAX" ( Beckman Co.) Ultracentrifugation at 45,000 rpm (about 100,000 g) for 15 minutes at 4°C (rotor: MLA-130, centrifuge tube: thick-walled heteromorphic polymer Tube (Polyallomer Tube)). Next, the uppermost chylomicron fraction was removed from the ultracentrifuged plasma, and then 180 μL of the product name "(registered trademark) Optiprep" was added and mixed to 720 μL of the plasma...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap