Aspirin suppository and preparation method thereof
A technology of aspirin and suppositories, which is applied in the field of pharmaceutical preparations, can solve the problems of low dissolution rate and low bioavailability, and achieve the effects of reducing toxicity and side effects, improving safety, and reducing dust flying
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
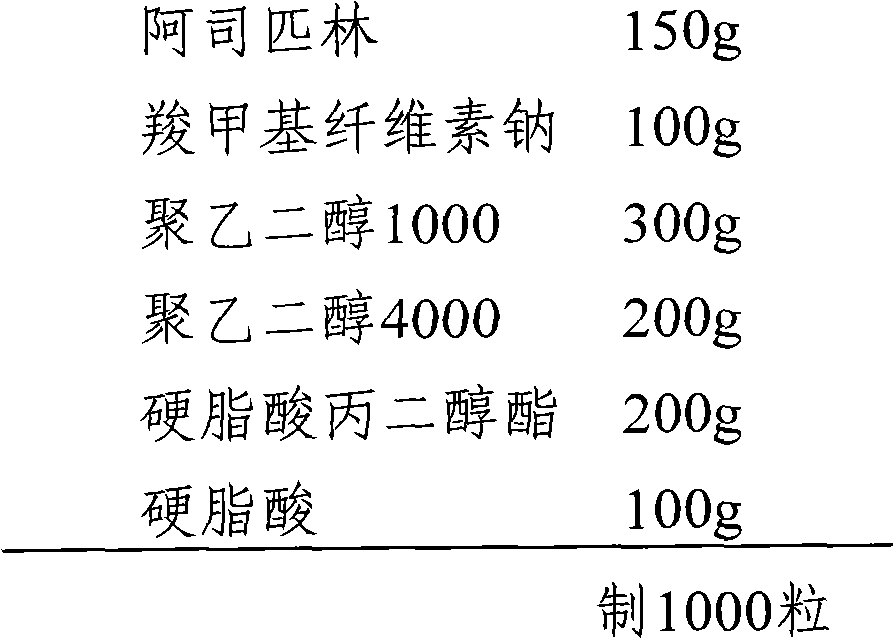
[0015] The preparation of embodiment 1 aspirin suppositories
[0016]
[0017] (1) Sieve 150g of aspirin and 100g of sodium carboxymethylcellulose through 100 mesh and mix well.
[0018] (2) 500 g of polyethylene glycols (300 g of polyethylene glycol 1000 and 200 g of polyethylene glycol 4000) were melted in a water bath at 85°C.
[0019] (3) Add propylene glycol stearate and stearic acid into (2), and stir well.
[0020] (4) Add (1) into (3) and stir evenly to prepare a suppository paste filling.
[0021] (5) When the temperature in (4) drops below 40°C, pour it into the mold, after cooling and solidifying, cut off the overflowing part with a knife, open the plug mold, and push it out.
Embodiment 2
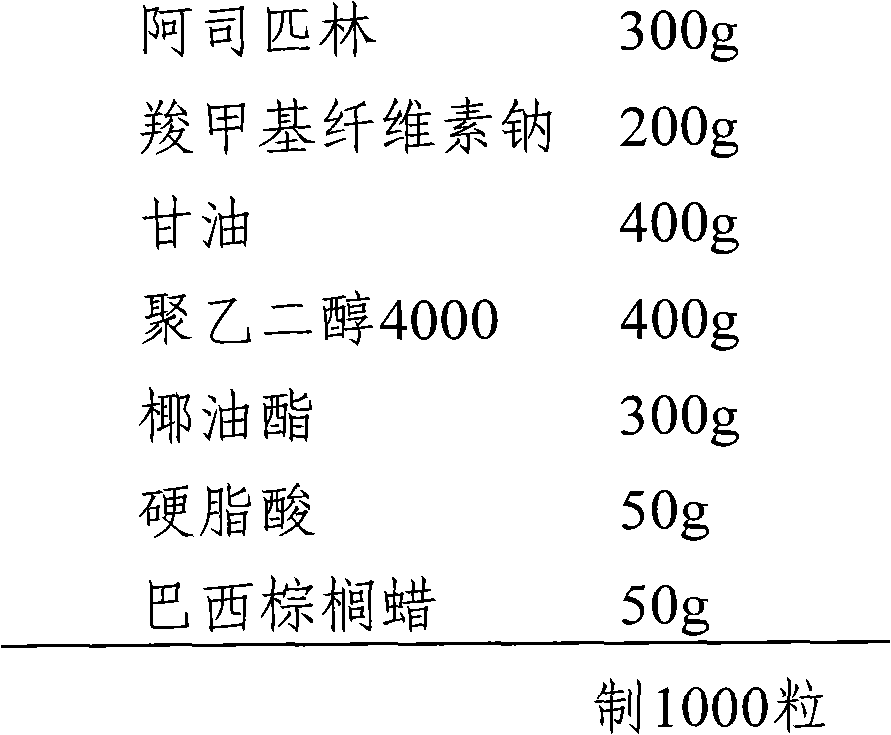
[0022] The preparation of embodiment 2 aspirin suppositories
[0023]
[0024] (1) Sieve 300g of aspirin and 200g of sodium carboxymethylcellulose through 100 mesh and mix well.
[0025] (2) Melt 400 g of polyethylene glycol 4000 and 400 g of glycerin in a water bath at 85°C.
[0026] (3) Add coconut oil ester, stearic acid and carnauba wax to (2), and stir well.
[0027] (4) Add (1) into (3) and stir evenly to prepare a suppository paste filling.
[0028] (5) When the temperature in (4) drops below 40°C, pour it into the mold, after cooling and solidifying, cut off the overflowing part with a knife, open the plug mold, and push it out.
Embodiment 3
[0029] The preparation of embodiment 3 aspirin suppositories
[0030]
[0031] (1) Sieve 150 g of aspirin and 110 g of sodium carboxymethyl cellulose through 100 mesh and mix well.
[0032] (2) Melt 400g of polyethylene glycol 1000 and 300g of gelatin in a water bath at 85°C.
[0033] (3) Add the gooseberry oil ester and stearic acid into (2), and stir well.
[0034] (4) Add (1) into (3) and stir evenly to prepare a suppository paste filling.
[0035] (5) When the temperature in (4) drops below 40°C, pour it into the mold, after cooling and solidifying, cut off the overflowing part with a knife, open the plug mold, and push it out.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com