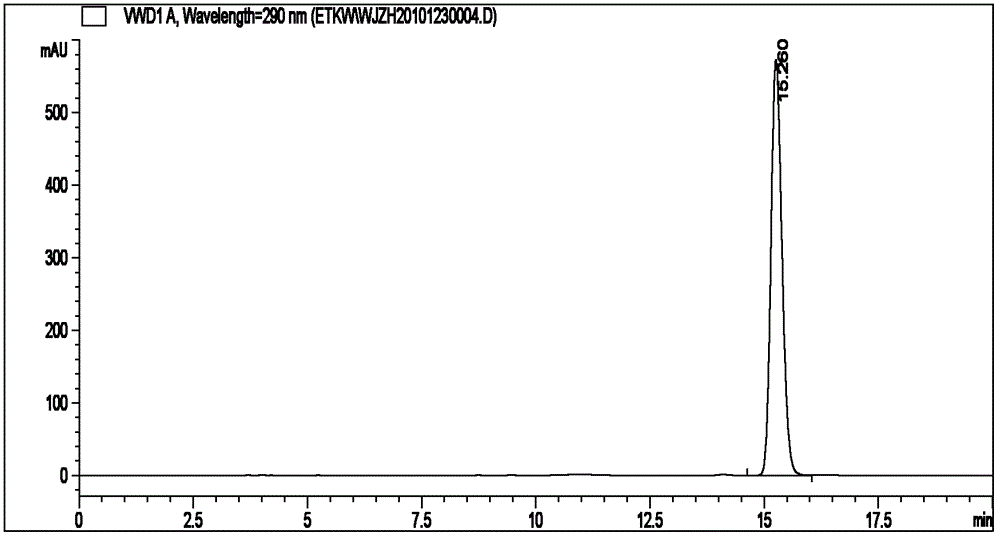
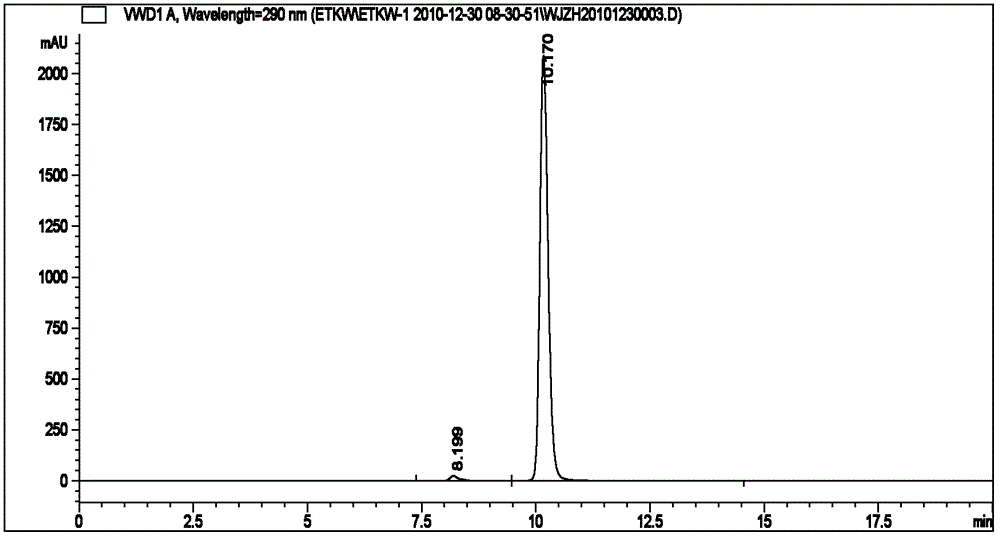
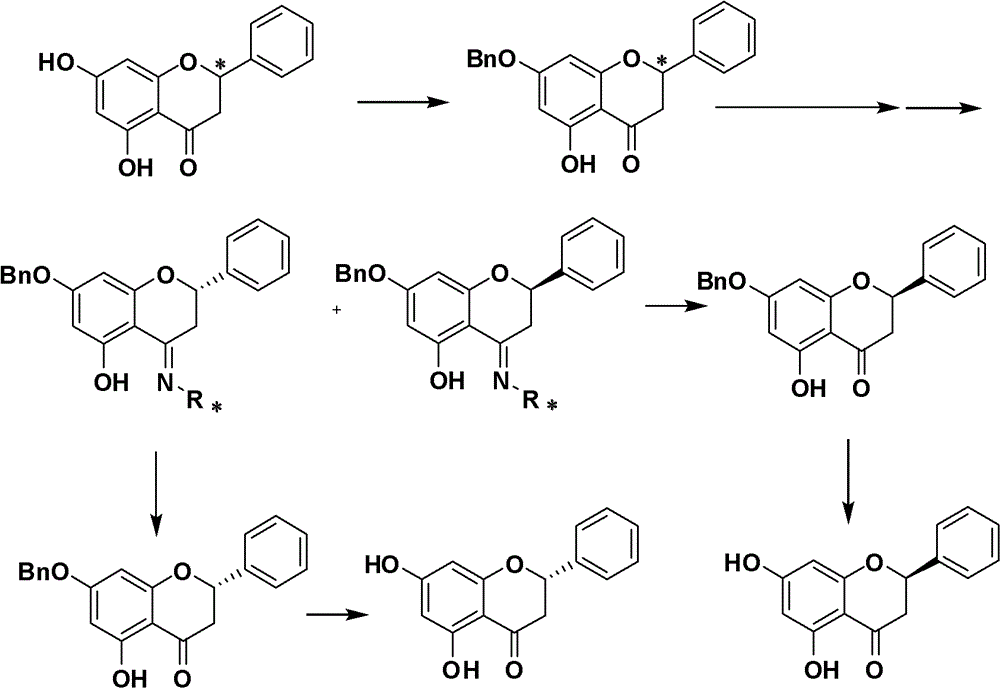
Method for synthetizing (R)-(plus)-pinocembrin and (S)-(minus)-pinocembrin
A technology of pinoxerine and compounds, which is applied in the field of chemical drugs for the treatment of cardiovascular and cerebrovascular diseases, and can solve problems that have not been reported in the literature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation of embodiment 1 (S)-3-hydroxyl-N-methoxy-N-methyl-3-phenylpropanamide (3)
[0030] Dissolve 66.9 g (0.672 mol) of N, O-dimethylhydroxylamine hydrochloride in 1200 mL of anhydrous THF, and add 840 mL of n-BuLi (1.6 mol·L -1 , 0.134mol), after dropping, stir at room temperature for 15min, then lower the temperature to -78°C, add dropwise a solution of 43.4g (0.224mol) of compound (2) in THF (300mL), dropwise, continue to react for 2h, with saturated ammonium chloride The reaction was quenched by the solution, extracted with ethyl acetate, dried over anhydrous sodium sulfate, evaporated to dryness, and obtained a colorless oily substance (S)-3-(tert-butyldimethylsilyloxy)-N-methyl Oxy-N-methyl-3-phenylpropanamide (3) 43.0 g, yield 93%.
[0031] 1 H NMR (CDCl 3 )δ (ppm): 2.81 (m, 2H), 3.20 (s, 3H), 3.62 (s, 3H), 4.26 (s, 1H), 5.15 (d, 1H, J=2.5and 9.2Hz,), 7.26 -7.42 (m, 5H).
Embodiment 2
[0032] The preparation of embodiment 2 (S)-3-(tert-butyldimethylsilyloxy)-N-methoxy-N-methyl-3-phenylpropanamide (4)
[0033] Dissolve compound (3) (20.9g, 100mmol) in 500mL of dichloromethane, add 13.6g (200mmol) of imidazole, 16.5g (110mmol) of tert-butyldimethylchlorosilane, stir overnight at room temperature, pour into saturated ammonium chloride solution, extracted with dichloromethane, combined extracts, dried over anhydrous sodium sulfate, evaporated to dryness, and obtained colorless oily substance (S)-3-(tert-butyldimethylsilyloxy)- N-methoxy-N-methyl-3-phenylpropanamide (4) 28.7 g, yield 89%.
[0034] 1 H NMR (CDCl 3 )δ(ppm): -0.13(s, 3H), 0.10(s, 3H), 0.84(s, 9H), 2.47(dd, 1H, J=3.5, 14.5Hz), 3.02-3.07(brs, 1H) , 3.18(s, 3H), 3.64(s, 3H), 5.26(dd, 1H, J=3.5, 9.0Hz), 7.24-7.39(m, 5H).
[0035] FAB-MS 341.2 (M+NH 4 + , 100%);
Embodiment 33
[0036] Embodiment 33, the preparation of 5-dimethoxyphenol (6)
[0037] Dissolve 126.0 g (1 mol) of phloroglucinol in 1000 mL of anhydrous methanol, slowly add 126.0 g of concentrated sulfuric acid dropwise under vigorous stirring, after the drop is completed, heat and reflux for 24 hours, cool to room temperature, evaporate the remaining methanol to dryness, and wash with a large amount of water to neutrality, extracted with ethyl acetate, dried over anhydrous sodium sulfate, evaporated to dryness, and obtained 101.0 g of a colorless oily substance 3,5-dimethoxyphenol (6) through silica gel column chromatography, with a yield of 65.6%.
[0038] 1 H NMR (CDCl 3) δ (ppm): 3.74 (s, 6H), 5.53 (brs, 1H), 6.03 (d, 2H, J=2.0Hz), 6.08 (t, 1H, J=2.0Hz).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com