Analysis detection method of 2-(tert-butyldimethylsilyl oxyl)ethanol
A technology of tert-butyldimethylsilyloxy and detection method, which is applied in the field of high performance liquid chromatography analysis, and can solve the problems of inapplicability to the analysis and detection of 2-(tert-butyldimethylsilyloxy)ethanol and the like , to achieve stable and reliable results, simple operation and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
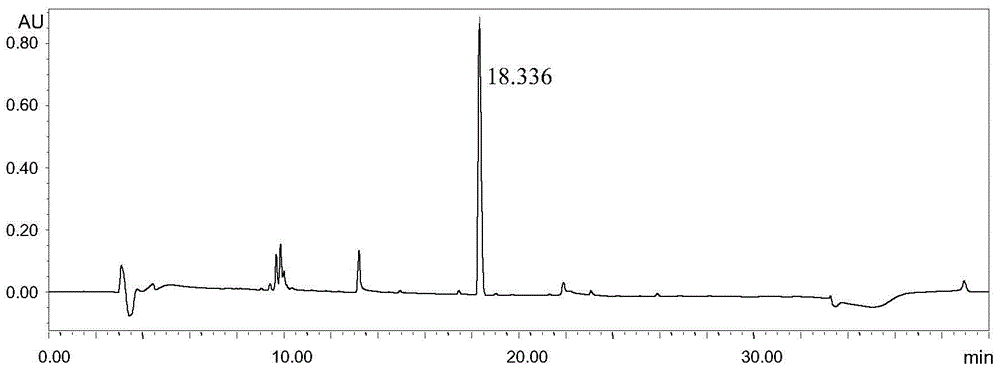
Embodiment 1
[0031] Instruments and conditions: Waters e2695 liquid chromatography system, Waters 2489 UV / visible light detector, chromatographic column: Shimadzu Inertsil ODS-3V; C18, 4.6×250mm, 5μm, detection wavelength 205nm, column temperature 25°C, flow rate 1.0mL / min, mobile phase: A-0.01% formic acid in water, B-0.01% formic acid in acetonitrile, gradient elution settings:
[0032] time (minutes)
Mobile phase A(%)
Mobile phase B(%)
0
91
9
20
9
91
30
9
91
31
91
9
40
91
9
[0033] Experimental procedure: Dissolve 2-(tert-butyldimethylsilyloxy)ethanol in methanol and quantitatively dilute to make a solution containing 15 mg of 2-(tert-butyldimethylsilyloxy)ethanol per 1 mL. As the test solution, accurately measure 20 μL of the test solution and inject it into the liquid chromatograph, carry out high-performance liquid chromatography analysis according to the above conditions, and record the chromatogra...
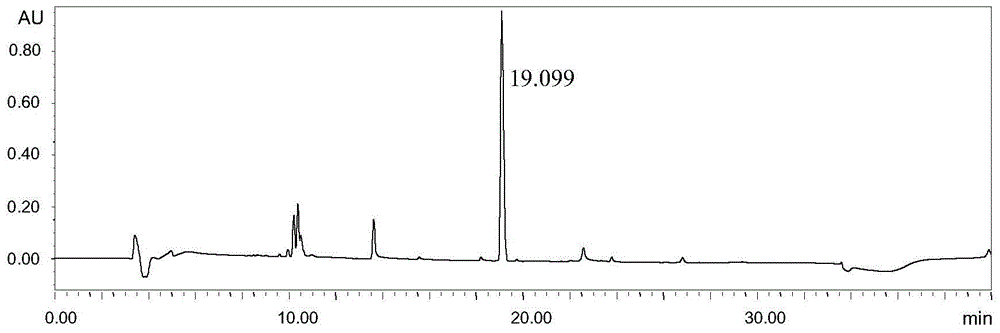
Embodiment 2
[0036] Instruments and conditions: Waters e2695 liquid chromatography system, Waters 2489 UV / visible light detector, chromatographic column: Shimadzu Inertsil ODS-3V; C18, 4.6×250mm, 5μm, detection wavelength 205nm, column temperature 20°C, flow rate 0.7mL / min, mobile phase: A-0.01% formic acid in water, B-0.01% formic acid in acetonitrile, gradient elution settings:
[0037] time (minutes)
Mobile phase A(%)
Mobile phase B(%)
0
88
12
20
12
88
30
12
88
31
88
12
40
88
12
[0038] Experimental procedure: Dissolve 2-(tert-butyldimethylsilyloxy)ethanol with acetonitrile and quantitatively dilute to make a solution containing 15 mg of 2-(tert-butyldimethylsilyloxy)ethanol per 1 mL. As the test solution, accurately measure 20 μL of the test solution and inject it into the liquid chromatograph, carry out high-performance liquid chromatography analysis according to the above conditions, and record the ...
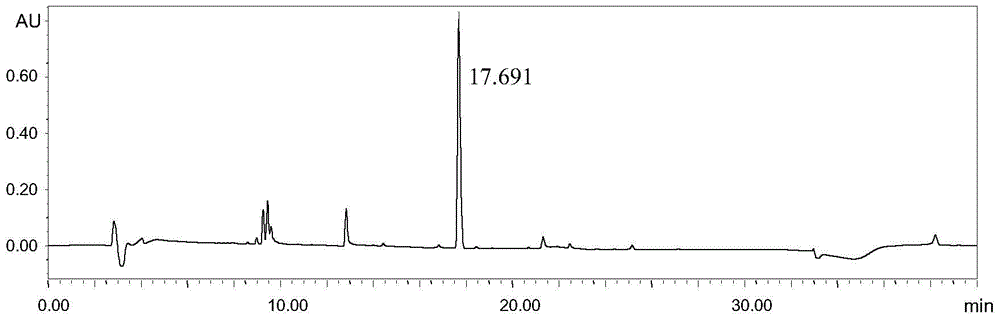
Embodiment 3
[0041] Instruments and conditions: Waters e2695 liquid chromatography system, Waters 2489 UV / visible light detector, chromatographic column: Shimadzu Inertsil ODS-3V; C18, 4.6×250mm, 5μm, detection wavelength 205nm, column temperature 30°C, flow rate 1.2mL / min, mobile phase: A-0.01% formic acid in water, B-0.01% formic acid in acetonitrile, gradient elution settings:
[0042] time (minutes)
Mobile phase A(%)
Mobile phase B(%)
0
97
3
20
3
97
30
3
97
31
97
3
40
97
3
[0043] Experimental procedure: Dissolve 2-(tert-butyldimethylsilyloxy)ethanol in methanol and quantitatively dilute to make a solution containing 15 mg of 2-(tert-butyldimethylsilyloxy)ethanol per 1 mL. As the test solution, accurately measure 20 μL of the test solution and inject it into the liquid chromatograph, carry out high-performance liquid chromatography analysis according to the above conditions, and record the chromatogra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com