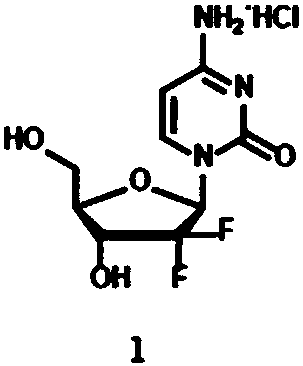
A kind of synthetic method of nucleoside compound
A compound and alkyl technology, which is applied in the field of drug synthesis, can solve problems such as low stereoselectivity, high dosage and concerns about drug toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Preparation of compound α-1-bromo-2-deoxy-2,2-difluoro-D-erythro-pentafuranose-3,5-dibenzoate
[0051] According to the literature (Howell, et.al., Journal of Organic Chemistry 1988, v.p.85-88) method, from the commercially available compound 2-deoxy-2,2-difluoro-D-erythro-pentofuranose-3 , 5-Dibenzyl-1-methanesulfonate is converted to the compound α-1-bromo-2-deoxy-2,2-difluoro-D-erythro-pentafuranosyl-3,5-benzidine esters. Concrete preparation method is as follows:
[0052] In the 100ml of chloroform was added at room temperature with 36g (3.0eq) of 33% hydrogen bromide acetic acid solution, and the reaction system was stirred at room temperature for 6 days. After the reaction was finished, 150 ml of water was added each time for washing, twice in total. Then wash with 150 ml of saturated aqueous sodium bicarbonate each time, twice in total. The organic phase was dried over anhydrous magnesium sulfate, and desolvated under reduced pressure to obtain a pale yello...
Embodiment 2
[0054] Preparation of 2-selenocytosine
[0055] 2-Chlorocytosine (2g, 15.4mmol) was added to the sodium selenohydride ethanol solution protected by argon (preparation process: selenium (3g, 38.0mmol) and sodium borohydride (1.75g, 46.2mmol) were added to absolute ethanol ( 100mL) at 10°C for 30 minutes), heated to reflux for 48 hours, cooled, added 10mL of water, filtered with suction, suspended the filter cake in 15ml of absolute ethanol, then added 220mg of sodium borohydride, stirred for 30 minutes, filtered with suction, and dried , an off-white solid (1.1 g) was obtained with a yield of 39%.
[0056] The analysis data is: 1 H-NMR(DMSO-d6)δ: 12.39(s,1H),7.69and 7.84(ss,2H),7.40(d,J=6.0Hz,1H),6.07(d,J=6.0Hz,1H); 13 C-NMR (DMSO-d6) δ: 175.7, 161.6, 142.6, 97.6; HRMS (ESI-TOF), C4H5N3Se, [M+Na]=197.9538 (calc.197.9546).
Embodiment 3
[0058] Preparation of 2-thiocytosine
[0059] Under nitrogen protection, add metal sodium (4.8g) to n-butanol (88mL) at 40-50°C, stir to dissolve, then add thiourea (16g) and 3,3-diethoxy Propionitrile (28g), heated to reflux for 5 hours, cooled to room temperature, adjusted pH to 7-8 with acetic acid, filtered to obtain a yellow solid, recrystallized with water to obtain light yellow 2-thiocytosine (12g), yield 48% .
[0060] analyze data: 1 H-NMR (600 MHz, DMSO-d6) δ: 11.94 (s, 1H), 7.54 (s, 2H), 7.39 (s, 1H), 5.91 (s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com