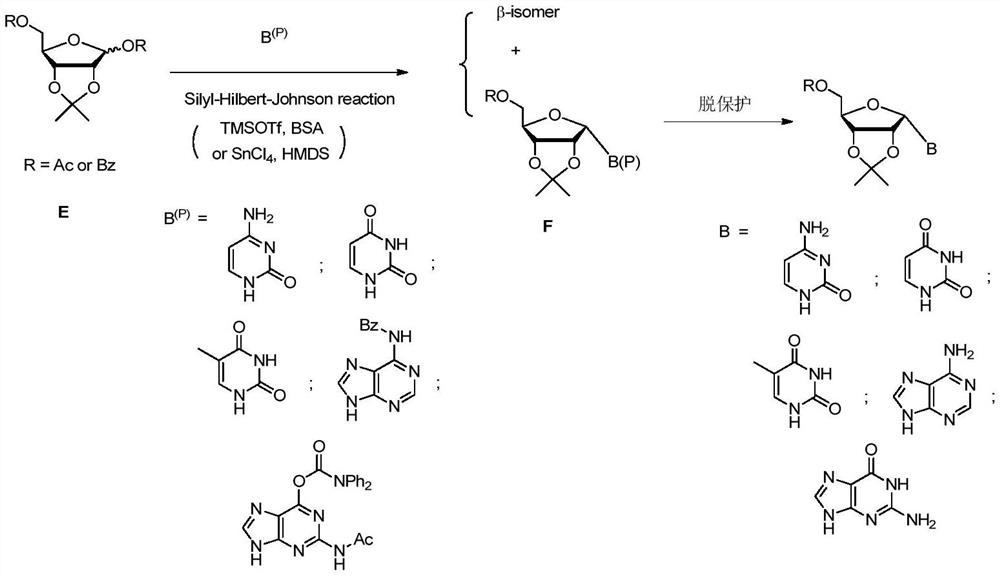
A kind of alpha nucleoside synthesis method
A technology for α-nucleosides and compounds, which is applied in the field of nucleoside compound synthesis, and can solve problems such as poor versatility and inapplicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
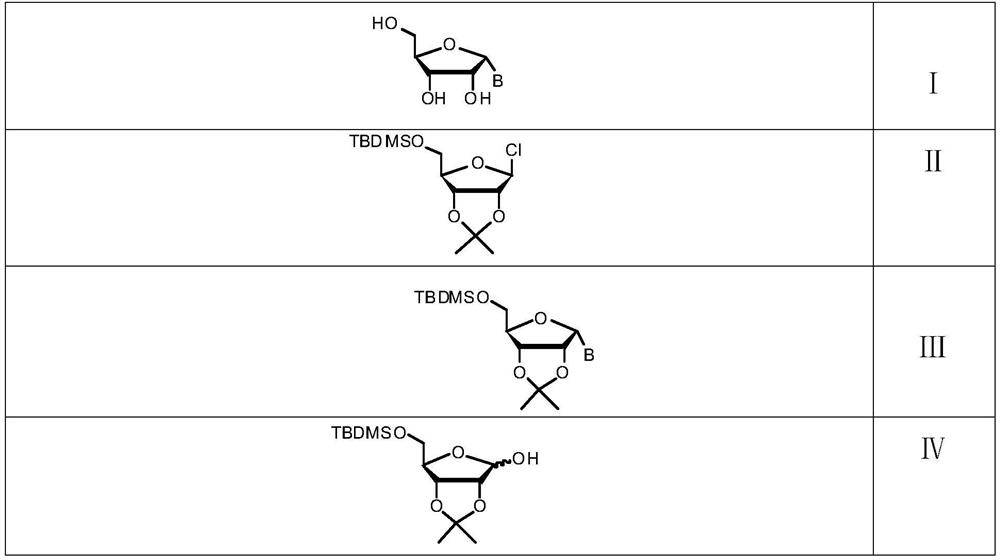
[0034] Specifically, the preparation method of the alpha nucleoside provided by the present invention comprises steps:
[0035] In the first step, the hydroxyl group on the raw material 5-O-tert-butyldimethylsilane-2,3-O-isopropylidene-D-ribofuranose is chlorinated to obtain the compound of formula II, and the reaction solution is directly For the next step reaction;
[0036] In the second step, under the action of an inorganic base, the reaction solution prepared from the compound of formula II is coupled with the base to obtain the compound of formula III;
[0037] The third step is to deprotect the compound of formula III under the action of a catalyst to obtain the alpha nucleoside with the structure shown in formula I.
[0038] The synthetic route is:
[0039]
[0040] The reaction in the above-mentioned first step is a solvent with tetrahydrofuran, carbon tetrachloride is a chlorination reagent, and three (dimethylamino) phosphine is a promotor; Carbon tetrachloride...
Embodiment 1
[0080] Synthesis of α-adenosine
[0081] (1) Synthetic compound A crude product
[0082] Concentrate 100g of 5-O-tert-butyldimethylsilane-2,3-O-isopropylidene-D-ribofuranose with acetonitrile to remove water several times, and control the moisture to be less than or equal to 300ppm; under the protection of argon, the 5 -O-tert-butyldimethylsilane-2,3-O-isopropylidene-D-ribofuranose was dissolved in 1000mL THF, the air was replaced 3 times, the temperature of the system was lowered to -70±5°C, and the 75.8g of carbon tetrachloride; 69.6g of tris(dimethylamino)phosphine was added dropwise to the reaction solution, and the addition temperature was controlled at -70±5°C. The temperature of the reaction solution was controlled at -70±5°C and stirred for 1 hour; after 1 hour, the reaction solution was warmed to -40±3°C and stirred for 2 hours to obtain a reaction solution containing compound II, which was designated as reaction solution 1. Under the protection of argon, suspend 41...
Embodiment 2
[0088] Synthesis of α-guanosine
[0089] (1) Synthetic compound C crude product
[0090] Concentrate 100g of 5-O-tert-butyldimethylsilane-2,3-O-isopropylidene-D-ribofuranose with acetonitrile to remove water several times, and control the moisture to be less than or equal to 300ppm; under the protection of argon, the 5 -O-tert-butyldimethylsilane-2,3-O-isopropylidene-D-ribofuranose was dissolved in 1000mL THF, the air was replaced 3 times, the temperature of the system was lowered to -70±5°C, and the 75.8g of carbon tetrachloride; 69.6g of tris(dimethylamino)phosphine was added dropwise to the reaction solution, and the addition temperature was controlled at -70±5°C. The temperature of the reaction solution was controlled at -70±5°C and stirred for 1 hour; after 1 hour, the reaction solution was warmed to -40±3°C and stirred for 2 hours to obtain a reaction solution containing compound II, which was designated as reaction solution 1. Under the protection of argon, suspend 45...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com