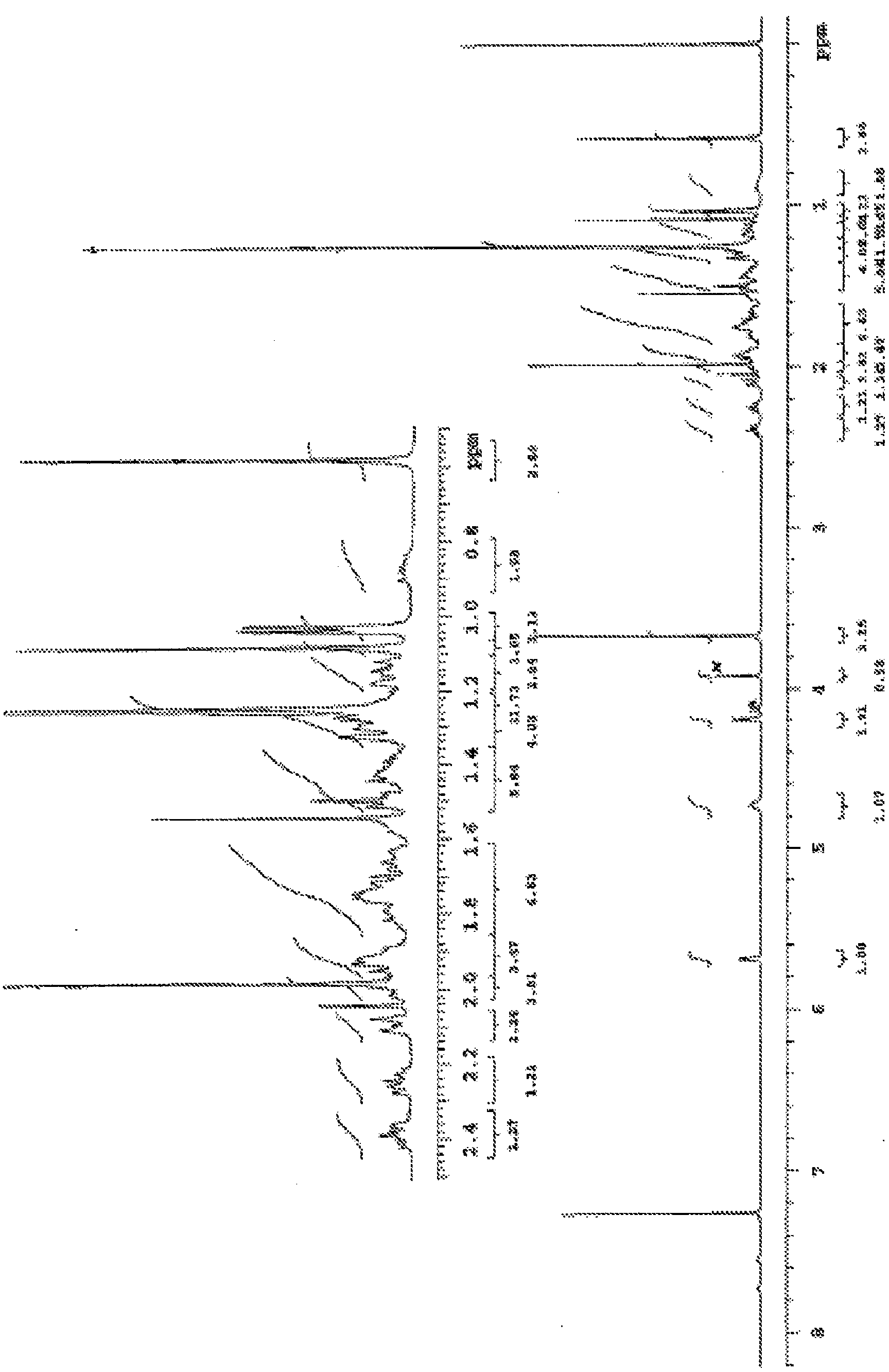
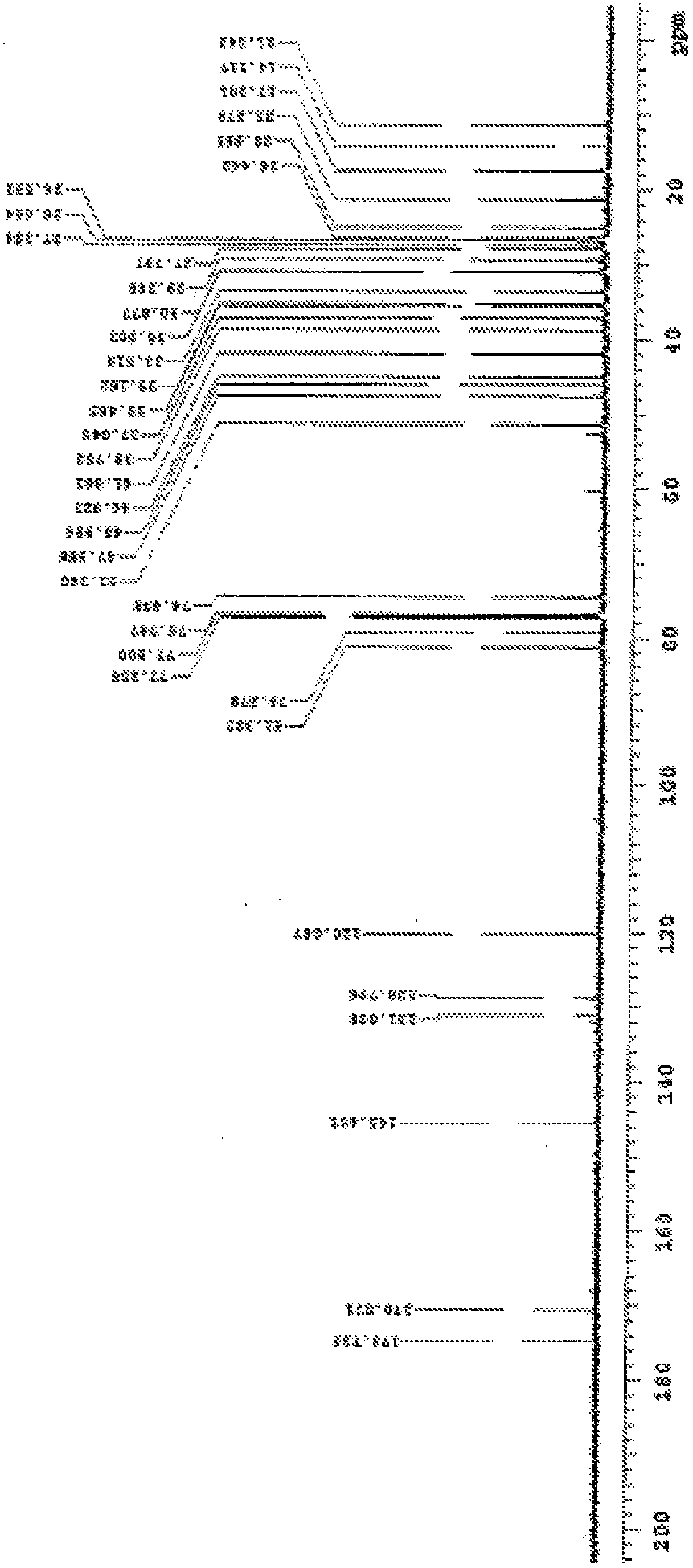
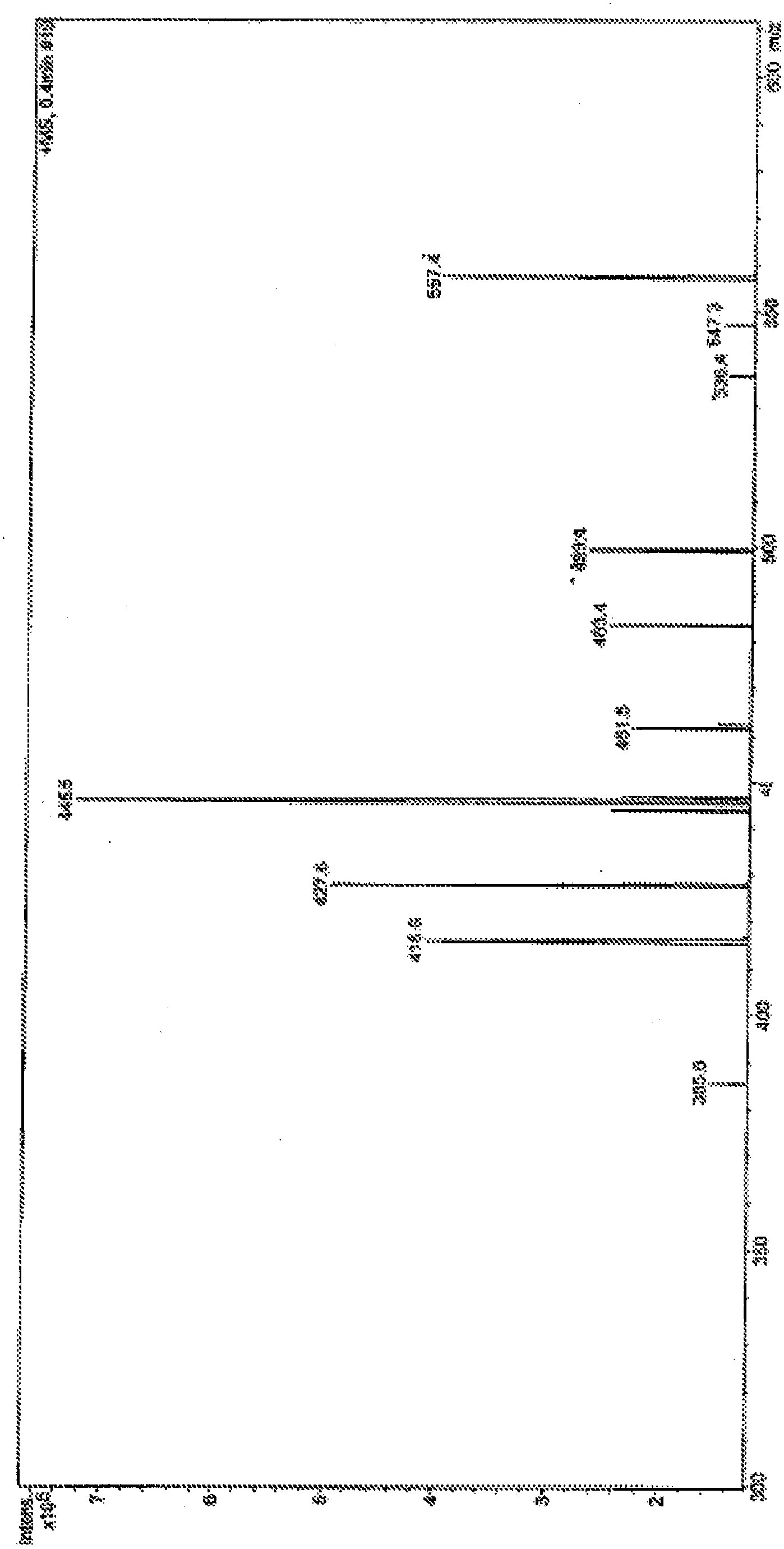
Methods For The Purification Of Deoxycholic Acid
A technology of bile acid and glycocholic acid, which is applied in the fields of chemical instruments and methods, organic chemistry, pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0549] In the examples below and elsewhere in the specification, the following abbreviations have the indicated meanings. If an abbreviation is not defined, it has the accepted meaning.
[0550] Ac 2 o
ACN
AcOH
CAD
CONC
strong
CrO 3
DCA
deoxycholic acid
DCM (CH 2 Cl 2 )
DMAP
4-Dimethylaminopyridine
DMF
EtAlCl 2
Ethyl aluminum dichloride
EtOAc
H or h
Hour
h 2 SO 4
HCl
HClO 4
HPLC
HPLC
[0551] HPLC-RI
High Pressure Liquid Chromatography with Refractive Index Detection
Hz
KBr
K-O t Bu
Potassium tert-butoxide
L...
example 1
[0556] Synthesis of 3α-Acetoxy-5β-Androstane-9,11-en-17-one from Hydrocortisone (36)
[0557] step 1
[0558]
[0559] To a solution of hydrocortisone (25.0 g) in DMF (150 mL) was added 10% Pd / C (1.5 g, 6-wt%) and the resulting slurry was autoclaved (60 psi) at 25-35 °C Hydrogenated for 6 hours. After the complete disappearance of the starting material was demonstrated by TLC (30% EtOAc in DCM), the The crude reaction mixture was filtered over a (8 g) bed and washed with DMF (100 mL). The solvent was completely removed by vacuum distillation below 65 °C to afford compound 15 (23.0 g, 91.5%) as a white solid.
[0560] step 2
[0561]
[0562] To a solution of compound 15 (23.0 g) in ethanol (350 mL) and DCM (350 mL) was added sodium borohydride (2.4 g), and the resulting solution was stirred at 25-35 °C for 3 hours. At this point, 50% acetone in water (200 mL) was added to quench excess reagent, followed by sodium periodate (33.7 g). The resulting solution was stir...
example 2
[0570] (Z)-3α-Acetoxy-5β-pregna-9(11), 17(20)-diene(30):
[0571]
[0572] Compound 30 can be prepared by converting compound 28 to compound 30 using a procedure similar to that described in Example 8.
[0573] Methods and examples of preparation and purification of DCA from compound 60 are described in GB2452358 and U.S. provisional application 61 / 288,132 entitled "METHODS FOR THE PURIFICATION OF DEOXYCHOLIC ACID" filed on December 18, 2009 , both of which are incorporated herein by reference in their entirety.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Dry weight | aaaaa | aaaaa |
| Dry weight | aaaaa | aaaaa |
| Dry weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com