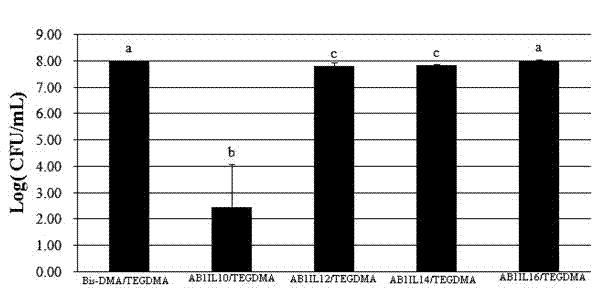
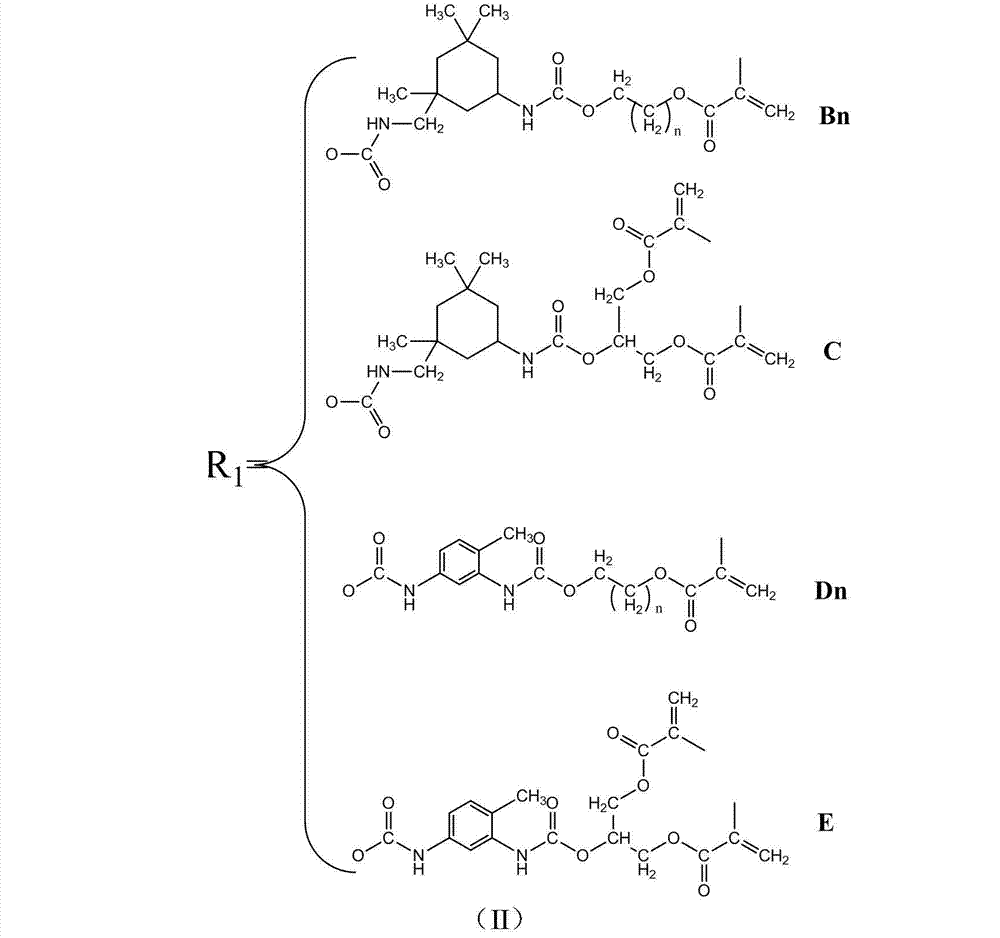
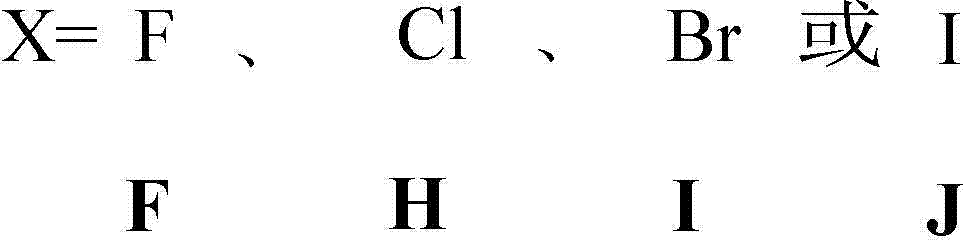Quaternary ammonium salt and carbamate structure containing antibacterial methyl acrylate monomer, its preparation method and application thereof
A technology of methacrylate and carbamate, which is applied to the antibacterial methacrylate monomer containing quaternary ammonium salt and carbamate structure and its preparation and application fields, and can solve the problem of the exudation of antibacterial monomer , limited cross-linking rate, difficult mixing, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 AB 1 IL 10 Monomer
[0040] This example AB 1 IL 10 The preparation method of monomer comprises the steps:
[0041]Step 1: Add 5.95g of N-methyldiethanolamine and 15.55g of 1-bromododecane into a three-necked flask equipped with magnets, add 100ml of dichloromethane, reflux and stir at 60°C for 15 hours, and cool to room temperature, filtered, and vacuum-dried the filter cake at 50°C for 72 hours to obtain N-methyl-N-dodecyldiethanolamine bromide.
[0042] Step 2: Add 4.44g of isophorone diisocyanate and 100ml of dichloromethane into a 250ml three-neck bottle equipped with a magnet, and continuously add 3.67g of N-methyl-N-dodecyl diisocyanate while stirring After reacting ethanolamine bromide and 0.002g dibutyltin dilaurate in an oil bath at 70°C for 8 hours, add 2.60g hydroxyethyl methacrylate and 0.0107g hydroquinone and continue the reaction in an oil bath at 75°C for 20 hours , and then purify the reaction product. The product was characterized by ...
Embodiment 2
[0044] Example 2 AB 1 IL 12 Monomer
Embodiment AB1
[0045] This embodiment AB 1 IL 12 The preparation method of monomer comprises the steps:
[0046] Step 1: Add 5.95g of N-methyldiethanolamine and 14.56g of 1-bromotetradecane into a three-necked flask equipped with magnets, add 100ml of dichloromethane, reflux and stir at 80°C for 5 hours, and cool Return to room temperature, filter, and vacuum-dry the filter cake at 50°C for 72 hours to obtain N-methyl-N-tetradecyldiethanolamine bromide.
[0047] Step 2: Add 4.44g of isophorone diisocyanate and 100ml of dichloromethane into a 250ml three-neck bottle equipped with a magnet, and continuously add 3.95g of N-methyl-N-tetradecyl diisocyanate under stirring After reacting ethanolamine bromide and 0.11g dibutyltin dilaurate in an oil bath at 70°C for 8.5 hours, add 2.60g hydroxyethyl methacrylate and 0.066g hydroquinone and continue to react in an oil bath at 75°C for 22 hours , and then purify the reaction product. The product is characterized by infrared and NMR:
[0048] FT-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com