Preparation method for high-enantioselectivity synthesized (S)-omeprazole and salt thereof
An enantioselective, omeprazole technology, applied in the field of chemical synthesis of drugs, can solve the problems of large usage of chiral ligands, difficulty in industrial production, waste of omeprazole raw materials, etc., and achieve optical purity of the product. And the effect of high chemical purity and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
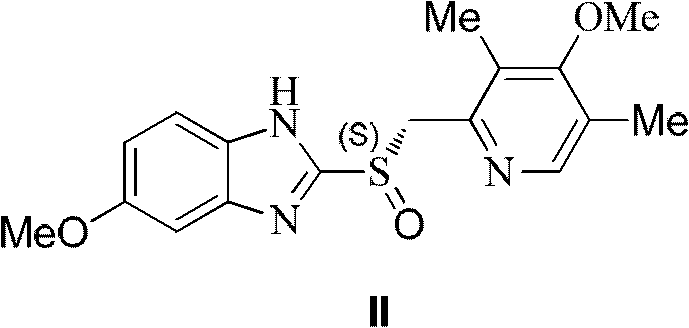
[0053] The preparation of embodiment 1 (S)-omeprazole
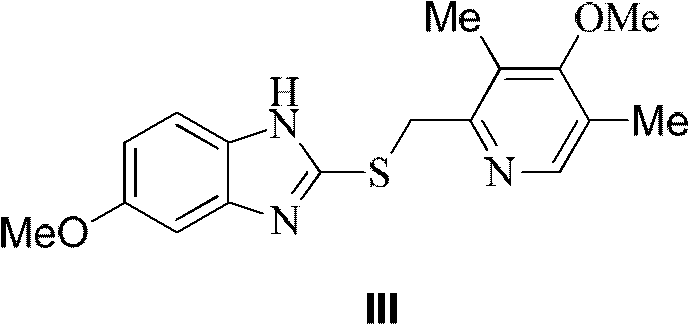
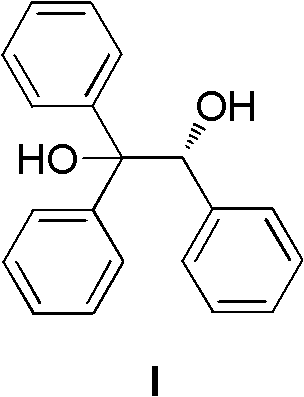
[0054] In a 500mL three-necked flask, omeprazole sulfide (32.9g, 100mmol) was suspended in toluene (150mL), and (R)-(+)-1,1,2-triphenyl-1,2- Ethylene glycol (5.8g, 20mmol) and water (0.18g, 10mmol) were reacted by heating at 55°C for 10 minutes. Tetraisopropyl titanate (5.8 g, 20 mmol) was added dropwise, and the reaction was continued at 55° C. for 50 minutes. Stop heating, cool to room temperature, add imidazole (0.68g, 10mmol), react for 5 minutes, then slowly add 80% cumene hydroperoxide (19.0g, 100mmol) dropwise, and stir at room temperature for 3 hours. After chromatographic analysis, the HPLC purity of the crude reaction product was 96%, and the ee value was 95%.
Embodiment 2
[0055] The preparation of embodiment 2 (S)-omeprazole
[0056] In a 500mL three-necked flask, omeprazole sulfide (32.9g, 100mmol) was suspended in toluene (150mL), and (R)-(+)-1,1,2-triphenyl-1,2- Ethylene glycol (5.8g, 20mmol) and water (0.18g, 10mmol) were reacted by heating at 55°C for 10 minutes. Tetraisopropyl titanate (5.8 g, 20 mmol) was added dropwise, and the reaction was continued at 55° C. for 50 minutes. Stop heating, cool to room temperature, add diisopropylethylamine (1.3g, 10mmol), react for 5 minutes, then slowly add 80% cumene hydroperoxide (19.0g, 100mmol) dropwise, and stir at 30°C 2 hours. After chromatographic analysis, the HPLC purity of the crude reaction product was 95%, and the ee value was 88%.
Embodiment 3
[0057] The preparation of embodiment 3 (S)-omeprazole
[0058] In a 500mL three-necked flask, omeprazole sulfide (32.9g, 100mmol) was suspended in toluene (150mL), and (R)-(+)-1,1,2-triphenyl-1,2- Ethylene glycol (5.8g, 20mmol) and water (0.18g, 10mmol) were reacted by heating at 30°C for 10 minutes. Tetraisopropyl titanate (5.8 g, 20 mmol) was added dropwise, and the heating reaction was continued at 30°C for 120 minutes. Stop heating, cool to room temperature, add imidazole (0.68g, 10mmol), react for 5 minutes, then slowly add 80% cumene hydroperoxide (19.0g, 100mmol) dropwise, and stir at room temperature for 3 hours. After chromatographic analysis, the HPLC purity of the crude reaction product was 86%, and the ee value was 52%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com