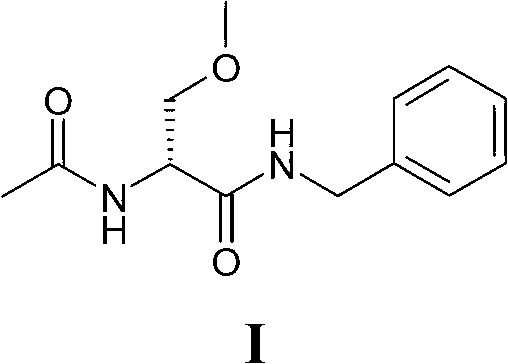
Process for the synthesis of lacosamide
A technology for synthesizing lacosamide and compounds, which is applied in the field of preparation of 2--N-benzyl-3-methoxypropionamide, can solve the undeveloped resolution of lacosamide racemic mixture, R enantiomer Isomer purification is complex and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
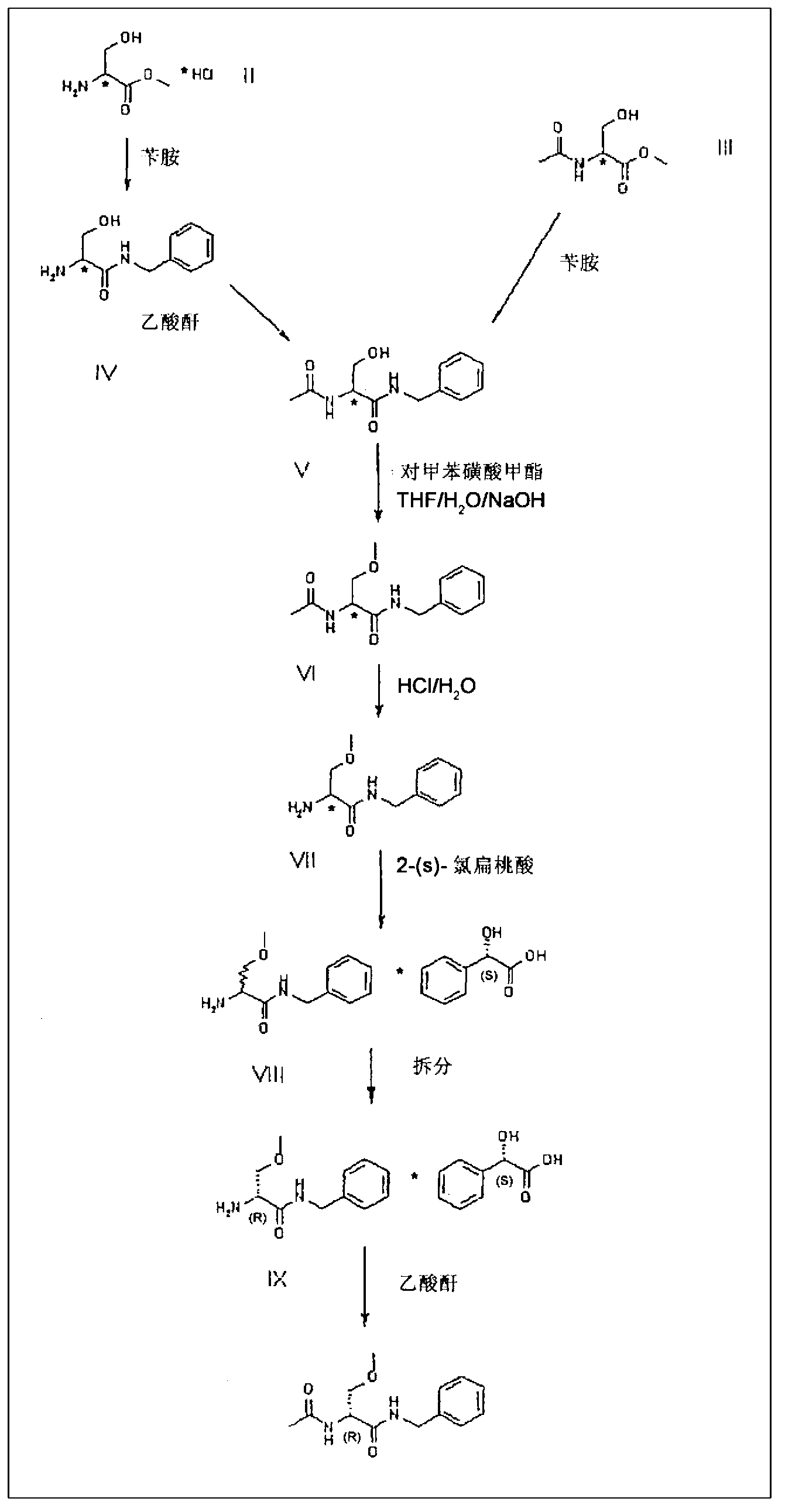
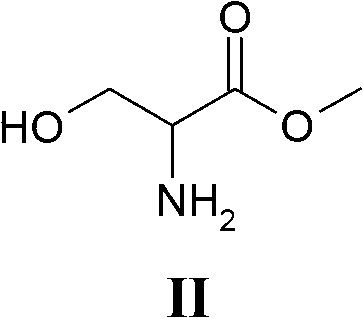
[0034] Example 1 (synthesis of V from III)
[0035] 100 g of compound III and 332 g of benzylamine were charged into a 1 liter reactor equipped with mechanical stirrer, reflux condenser, thermometer and placed under nitrogen atmosphere. The mixture was heated to 65° C. and kept stirring at this temperature for 12 hours. It was distilled under vacuum to remove excess benzylamine. It was cooled to about 55-60° C. and 50 ml of THF were added. It was then cooled to 20°C and the resulting solid was filtered off, which was then dried under vacuum at 40°C. 123.2 g of compound V are obtained.
[0036] 84% molar yield.
Embodiment 2
[0037] Embodiment 2 (synthetic VI from V)
[0038] 148 g of compound V, 7.4 g of tetrabutylammonium sulfate, 740 ml of THF, 350 g of methyl p-toluenesulfonate and 440 g of 20% sodium hydroxide were charged into a 3 Liter reactor and placed under nitrogen atmosphere. The mixture thus obtained was heated to 35°C and kept stirring at this temperature for 4 hours. It was then cooled to 20-25°C and 165 g of 28% ammonium hydroxide were added. It was cooled to 5°C and the pH was adjusted to 7 using hydrochloric acid.
[0039] It was distilled under vacuum to remove THF and diluted with 1 liter of water.
[0040] It was extracted 4 times with 500 ml of dichloromethane, then the organic phases were combined and distilled to a small volume. Dichloromethane was replaced with 600 ml of isopropyl acetate. The resulting suspension was filtered and the resulting solid was washed with isopropyl acetate and dried under vacuum at 40°C.
[0041]115 g of compound VI are obtained. 73% mol...
Embodiment 3
[0042] Embodiment 3 (synthesis of VIII from VI)
[0043] Legenda
[0044] Riflusso=reflux
[0045] 63.5 g of compound VI, 850 ml of water and 65 g of 37% HCl were charged into a 2 liter reactor with mechanical stirrer, reflux condenser, thermometer and inertized with nitrogen. The mixture was heated to reflux and kept stirring for 6 hours, then it was cooled to 20-25°C. The pH was corrected to 11.5 ± 0.5 using 30% sodium hydroxide. The resulting mixture was extracted twice with 300 ml of dichloromethane. The combined organic phases were concentrated to a small volume, 300 ml of ethyl acetate and 35 g of 2-(S)-chloromandelic acid were added. Half of the solvent was distilled off and allowed to stir at room temperature until precipitation was complete. The solid was filtered off and dried under vacuum at 40°C. 84.3 g of diastereomeric mixture VIII are obtained.
[0046] 84.1% molar yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com