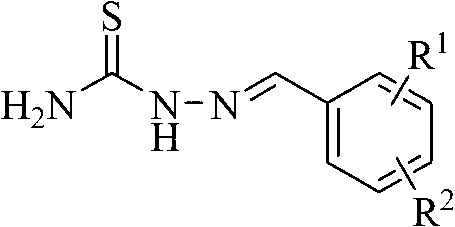
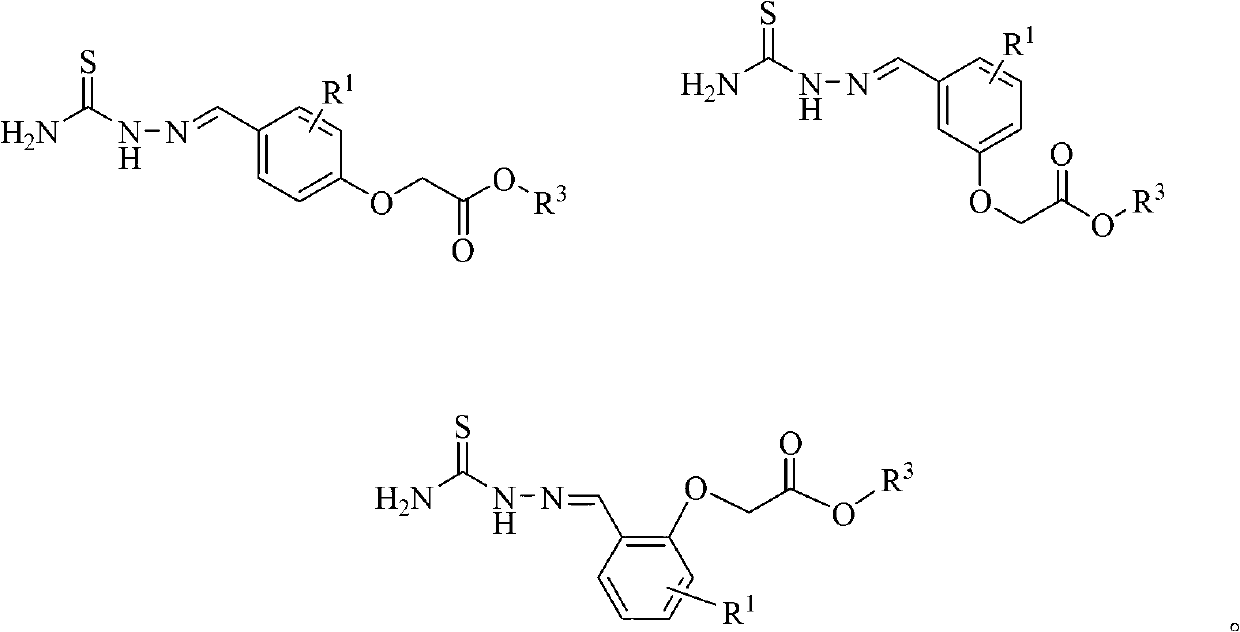
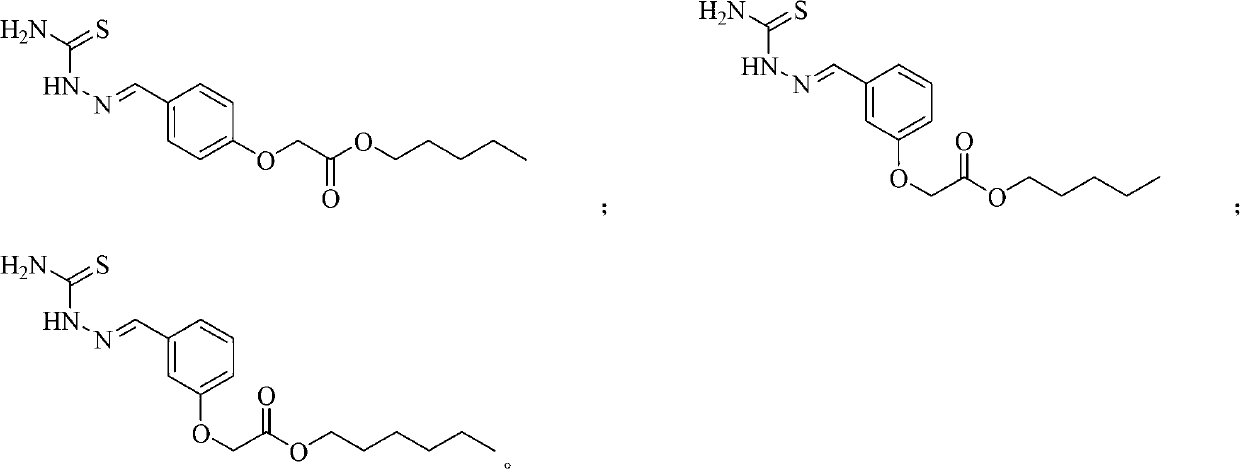
(Thiosemicarbazideformyl) phenoxyacetic acid derivative and application thereof
A technology of phenoxyacetic acid derivatives and thiosemicarbazide formal, which is applied in the fields of cosmetics, food, and medicinal chemistry, can solve the problems of low inhibitory activity, carcinogenicity, and high price, and achieve good tyrosinase inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Preparation of methyl 4-(thiosemicarbazide formal) phenoxyacetate (compound 1)
[0032] Dissolve 2.44g (20mmol) of p-hydroxybenzaldehyde in 40mL dry acetone, add 5.52g (40mmol) of potassium carbonate (microwave activation) and 3.24g (30mmol) of methyl chloroacetate, and stir for 8 hours under reflux conditions after addition. TLC tracks until the reaction is complete. Filter and spin dry the solvent to obtain the initial product. The intermediate was separated by silica gel column chromatography with a yield of 83%.
[0033] Dissolve 5 mmol of the intermediate obtained above in anhydrous methanol, add 5 mmol of thiosemicarbazide, and stir for 4 hours under reflux conditions. TLC will track until the reaction is complete. After the reaction solution is cooled, it is filtered to obtain a white solid product, namely methyl 4-(thiosemicarbazide formal) phenoxyacetate (1), with a yield of 92%. 1 H-NMR(300MHz,DMSO-d 6 ): δ11.37(s,1H,NH), 8.16(s,1H,NH 2 ),8.01(s,1H,NCH...
Embodiment 2
[0082] Example 2 In vitro tyrosinase inhibitory activity test of (thiosemicarbazide formal) phenoxyacetic acid derivatives
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com