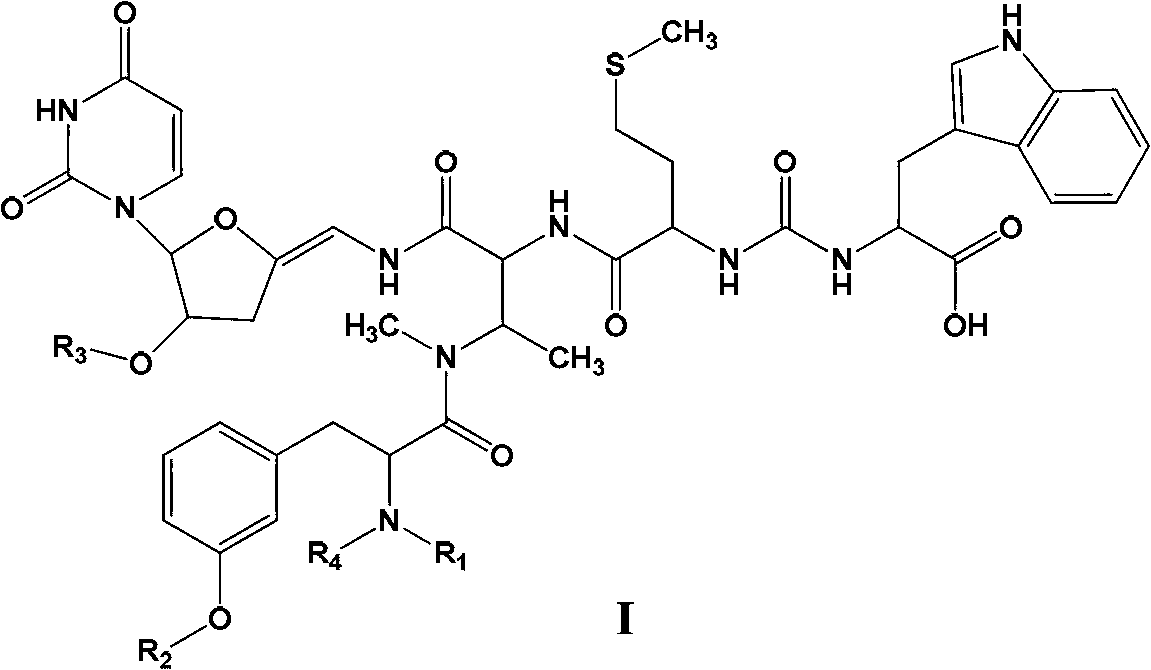
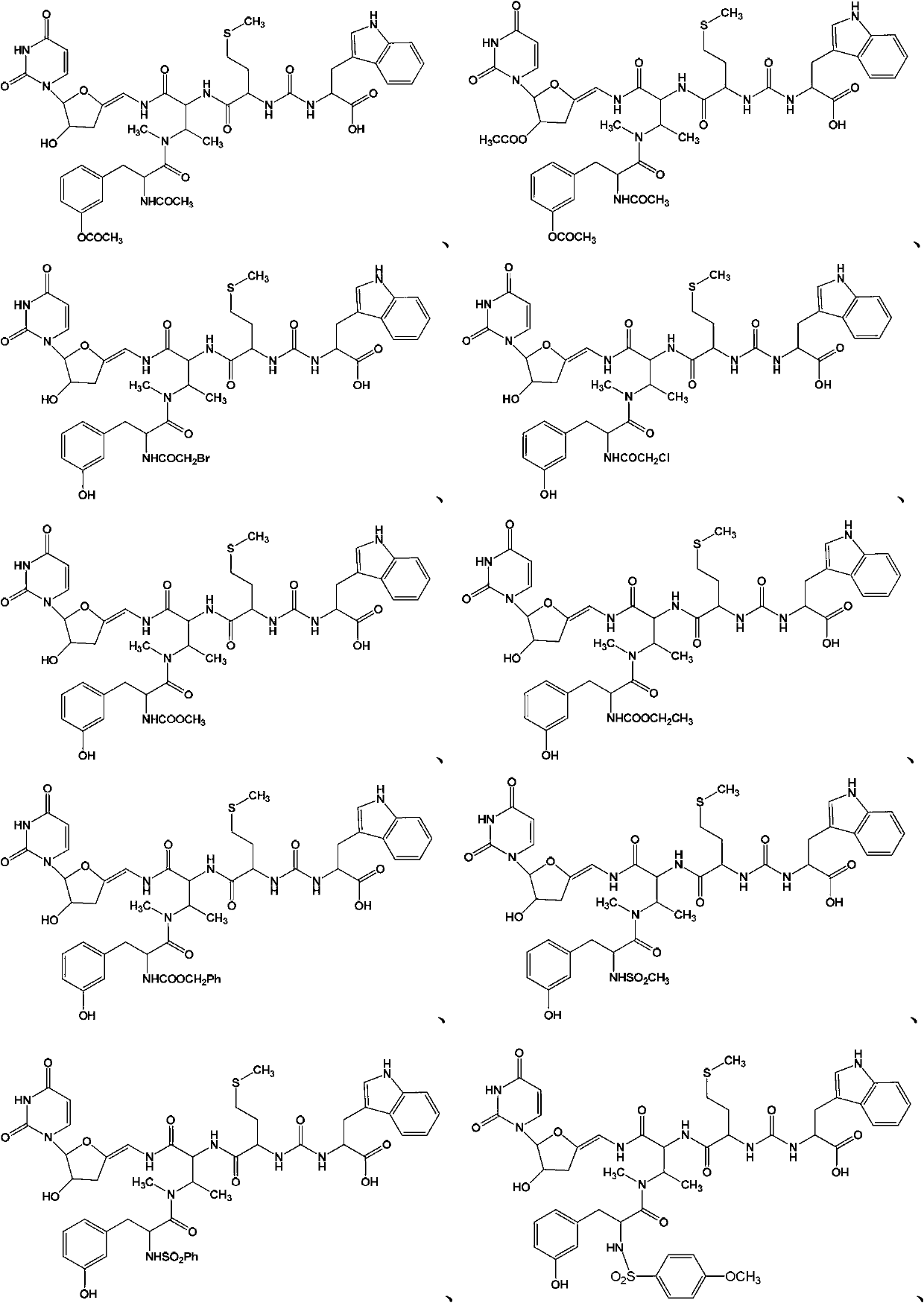
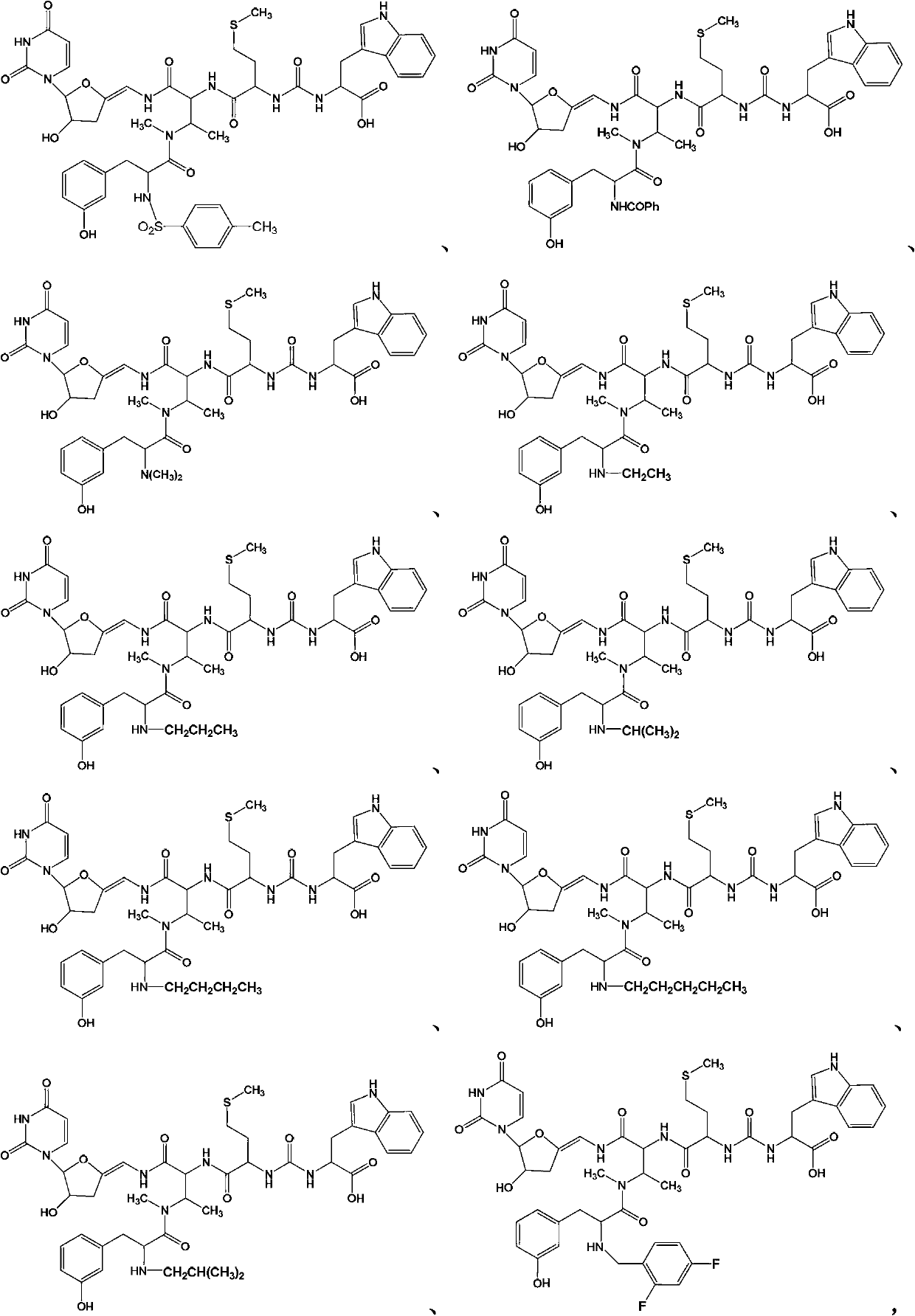
New sansanmycins, and their uses as anti-tuberculous medicines
A kind of drug, halogen technology, applied in the field of medicinal chemistry, can solve the problem of anti-tuberculosis candidates with no new structure skeleton, since the mid-1970s, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Example 1: Synthesis of SSA-1A
[0089] Dissolve 90mg (0.104mmol) of 50% SSA in 2ml of distilled water, add 20μl of acetic anhydride (0.208mmol), add 1mol / L NaOH solution to adjust the pH to about 6, and stir at room temperature for 3 hours. Purified by preparative liquid chromatography, a white powder 11.2mg was obtained, the yield was 23.73%, and the ESI(-) was 946.4. 1 H NMR(600M HZ,DMSO-d 6 ):δ1.72(3H,S,H N-CO-CH3 ), 2.24(3H,S,H Ar-O-CO-CH3 ), 4.73(1H,m,H m-Tyr-2 ), 6.92 (1H, S, H m-Tyr-2‘ ), 6.94 (1H, d, J=7.2HZ, H m-Tyr-4‘ ), 6.95 (1H, d, J=7.2HZ, H m-Tyr-6‘ ), 7.01 (1H, t, J=7.2HZ, H m-Tyr-5‘ ). The remaining signals are the same as the values of Sansanmycin reported in the literature (Yunying Xie, et al. The Journal of Antibiotics. 2007, 60(2):158-161). H m-Tyr-2 The chemical shift of m-Tyr shifted from δ3.90 to low-field to δ4.73, indicating that the free amino group was acylated; H-2', 4', and 6'on m-Tyr all shifted to low-field, and 5'to high-field The...
Embodiment 2
[0090] Example 2: Synthesis of SSA-1B
[0091] Dissolve 90mg (0.104mmol) of 50% SSA in 2ml of distilled water, add 40μl of acetic anhydride (0.417mmol), add 1mol / L NaOH solution to adjust the pH to about 6, and stir at room temperature for 5 hours. Purified by preparative liquid chromatography, 3.5 mg of white powder was obtained, the yield was 7.09%, and the ESI (-) was 988.4. 1 H NMR(600M HZ,DMSO-d 6 ):δ1.72(3H,S,H N-CO-CH3 ), 1.98 (3H, S, H R-O-CO-CH3 ), 2.24(3H,S,H Ar-O-CO-CH3 ), 4.73(1H,m,H m-Tyr-2 ), 5.37 (1H,m,H sugar-2 ), 6.95 (1H, S, H m-Tyr-2‘ ), 6.96 (1H, d, J=7.2HZ, H m-Tyr-4‘ ), 6.97 (1H, d, J=7.2HZ, H m-Tyr-6‘ ), 7.03 (1H, t, J=7.2HZ, H m-Tyr-5‘ ). Other signals are the same as Sansanmycin. Like SSA-1A, the change of m-Tyr signal can judge that the free amino group and the 3'phenolic hydroxyl group on m-Tyr are acylated; H sugar-2 The shift to the lower field indicates that the hydroxyl group on sugar-2 is acylated.
Embodiment 3
[0092] Example 3: Synthesis of SSA-2
[0093] Dissolve 90mg (0.104mmol) of 50% SSA in 2ml DMSO, add 45ul triethylamine (0.312mmol), add 18ul bromoacetyl bromide (0.208mmol), and stir at room temperature for 2 hours. Purified by preparative liquid chromatography to obtain white powder 5.7mg, yield 11.11%, ESI (-) is 982.3, 984.3, 1H NMR (500M HZ, DMSO-d6): δ 4.32 (2H, S, H-Br- CH2), 4.70 (1H, m, Hm-Tyr-2). Other signals are the same as Sansanmycin. The chemical shift of Hm-Tyr-2 shifted to δ4.70 to the low field, indicating that the free amino group was acylated.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com