Dexamethasone small-molecular hydrogel drug delivery system and preparation method thereof
A technology of dexamethasone and small molecular water, which is applied in the directions of drug combination, pharmaceutical formula, medical preparation containing active ingredients, etc., can solve the problems of poor biocompatibility, complex degradation products, difficult synthesis, etc. mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
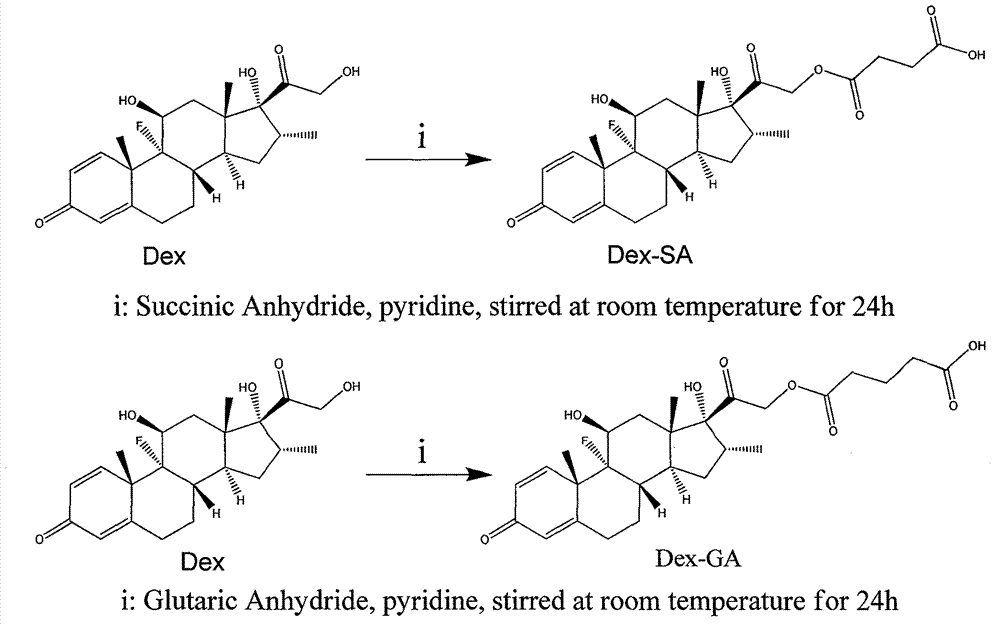
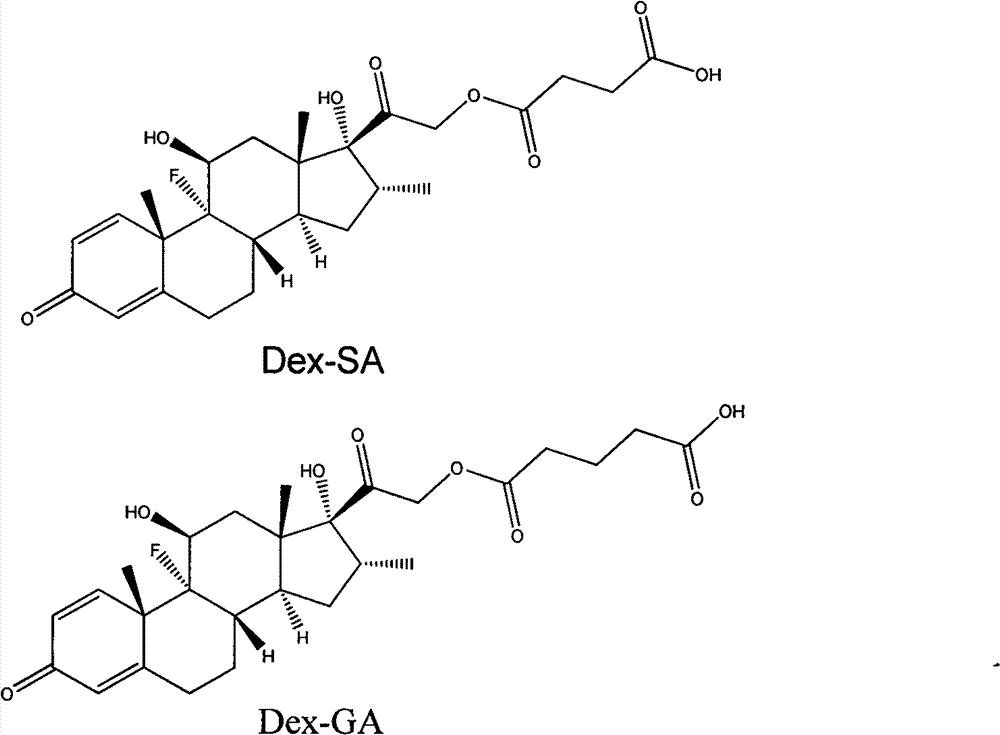
[0009] 1. Synthesis of Dexamethasone Derivatives
[0010] Put 1mmol of dexamethasone into a round bottom flask, add 4 equivalents of 1,4-succinic anhydride (glutaric anhydride), dissolve it with 12 milliliters of piperidine, stir at room temperature for 24 hours; then spin the piperidine to dryness ; Then add an appropriate amount of water, place it on ice to precipitate the product, then centrifuge (10000r / min) for 5-10min, discard the supernatant, wash with water for two changes; finally freeze-dry.
[0011] A: Dex-SA (dexamethasone succinate) NMR analysis
[0012] 1 HNMR (300MHz, DMSO-d 6 )δ7.27 (d, J=10.11, 1H), 6.19-6.24 (q, 1H), 5.99 (m, 1H), 5.42 (d, 1H), 5.17 (s, 1H), 4.99-5.06 (m, 1H), 4.75-4.82(m, 1H), 4.10-4.15(m, 1H), 2.82-2.91(m, 1H), 2.58-2.61(m, 3H), 2.26-2.40(m, 3H), 2.09- 2.18(m, 2H), 1.53-1.79(m, 6H), 1.05-1.096(m, 1H).MS: calc.M+=492.2, obsvd.(M+1) + =493.1.
[0013] B: Dex-GA (dexamethasone glutarate) NMR analysis
[0014] 1 HNMR (300MHz, DMSO-d 6 ...
example 1
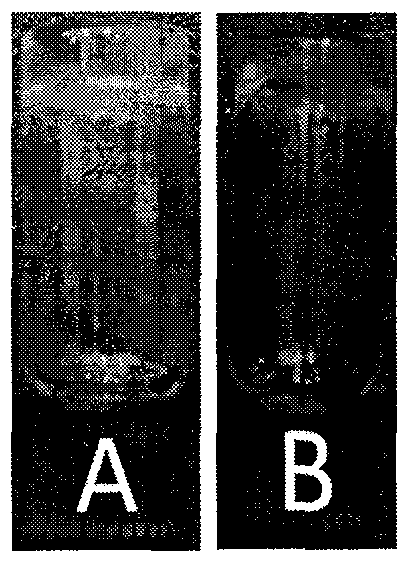
[0016] Example 1 (preparation of 2 wt% hydrogel at pH 7.0 and temperature 20°):
[0017] Take 4 mg of succinylated dexamethasone derivatives in a 1.5 ml glass bottle, add 150 microliters of PBS (PH=7.0) solution, adjust the pH value to 7.0 with sodium carbonate solution, and set the volume to 200 microliters with PBS solution, Stand still (20 degrees Celsius) to make it self-hydrolyzed, and form a gel after about 70 hours.
example 2
[0018] Example 2 (preparation of 2 wt% hydrogel at pH 7.0 and temperature 30°):
[0019] Take 4 mg of succinylated dexamethasone derivatives in a 1.5 ml glass bottle, add 150 microliters of PBS (PH=7.0) solution, adjust the pH value to 7.0 with sodium carbonate solution, and set the volume to 200 microliters with PBS solution, Stand still (30 degrees Celsius) to make it self-hydrolyzed, and form a gel after about 50 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com