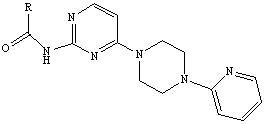
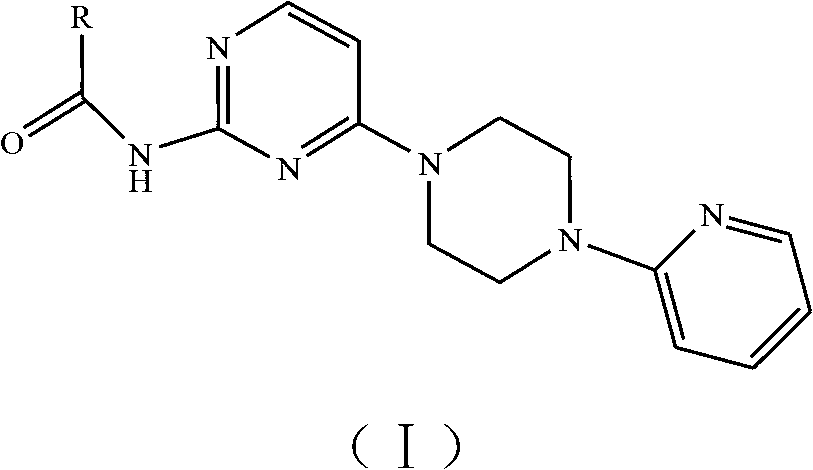
N-(4-(4-(pyridine-2-radical) piperazine-1-radical) pyrimidine-2-radical) amide and salt and preparation method and application thereof
A technology of pyridine and piperazine, applied in the field of chemical synthesis of drugs, can solve problems such as limited types and unsatisfactory cancer treatment effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1 N-(4-(4-(pyridin-2-yl)piperazin-1-yl)pyrimidin-2-yl)-2-chlorothiazole-5-amide compound
[0057] The preparation method of N-(4-(4-(pyridin-2-yl)piperazin-1-yl)pyrimidin-2-yl)-2-chlorothiazole-5-amide compound in this embodiment comprises the following steps:
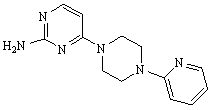
[0058] Step 1. Preparation of 2-amino-4-(4-(pyridin-2-yl)piperazine)pyrimidine
[0059] Add 0.6475g (5mmol) 2-amino-4-chloropyrimidine, 2.9340g (15mmol) 4-(pyridin-2-yl)piperazine, 9.5ml N, N-dimethylbenzylamine and 50ml of anhydrous pyridine were reacted at 20° C. for 24 hours under stirring, during which the degree of reaction was monitored by thin-layer chromatography. Filter after the reaction, the organic phase is first washed with 1M NaOH solution, then washed with distilled water until the organic phase is neutral, and finally anhydrous MgSO is added to the organic phase 4 Dry overnight, filter, after concentrating the filtrate, recrystallize with ethyl acetate, filter, collect the white powder ...
Embodiment 2
[0065] Example 2 Inhibitory effect of N-(4-(4-(pyridin-2-yl)piperazin-1-yl)pyrimidin-2-yl)amide compounds or pharmaceutically acceptable salts thereof on cancer cells
[0066] In this example, lung cancer cell line A549, liver cancer cell line Bel-7402, gastric cancer cell line SGC7901 and nasopharyngeal carcinoma cell line CNE2 were used as experimental subjects, and 2-amino-4-(4-(pyridin-2-yl)piper oxazine)pyrimidine, N-(4-(4-(pyridin-2-yl)piperazin-1-yl)pyrimidin-2-yl)-thiazole-5-amide, N-(4-(4-(pyridine- 2-yl)piperazin-1-yl)pyrimidin-2-yl)-2,4-dimethylthiazole-5-amide, N-(4-(4-(pyridin-2-yl)piperazine-1 -yl)pyrimidin-2-yl)-4-methylthiazole-5-amide, N-(4-(4-(pyridin-2-yl)piperazin-1-yl)pyrimidin-2-yl)-2 -Phenyl-4-methylthiazole-5-amide, N-(4-(4-(pyridin-2-yl)piperazin-1-yl)pyrimidin-2-yl)-2-(pyridine-3- Base)-4-methylthiazole-5-amide, N-(4-(4-(pyridin-2-yl)piperazin-1-yl)pyrimidin-2-yl)-2-(4-chlorophenyl )-4-methylthiazole-5-amide, N-(4-(4-(pyridin-2-yl)piperazin-1-yl)pyri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com