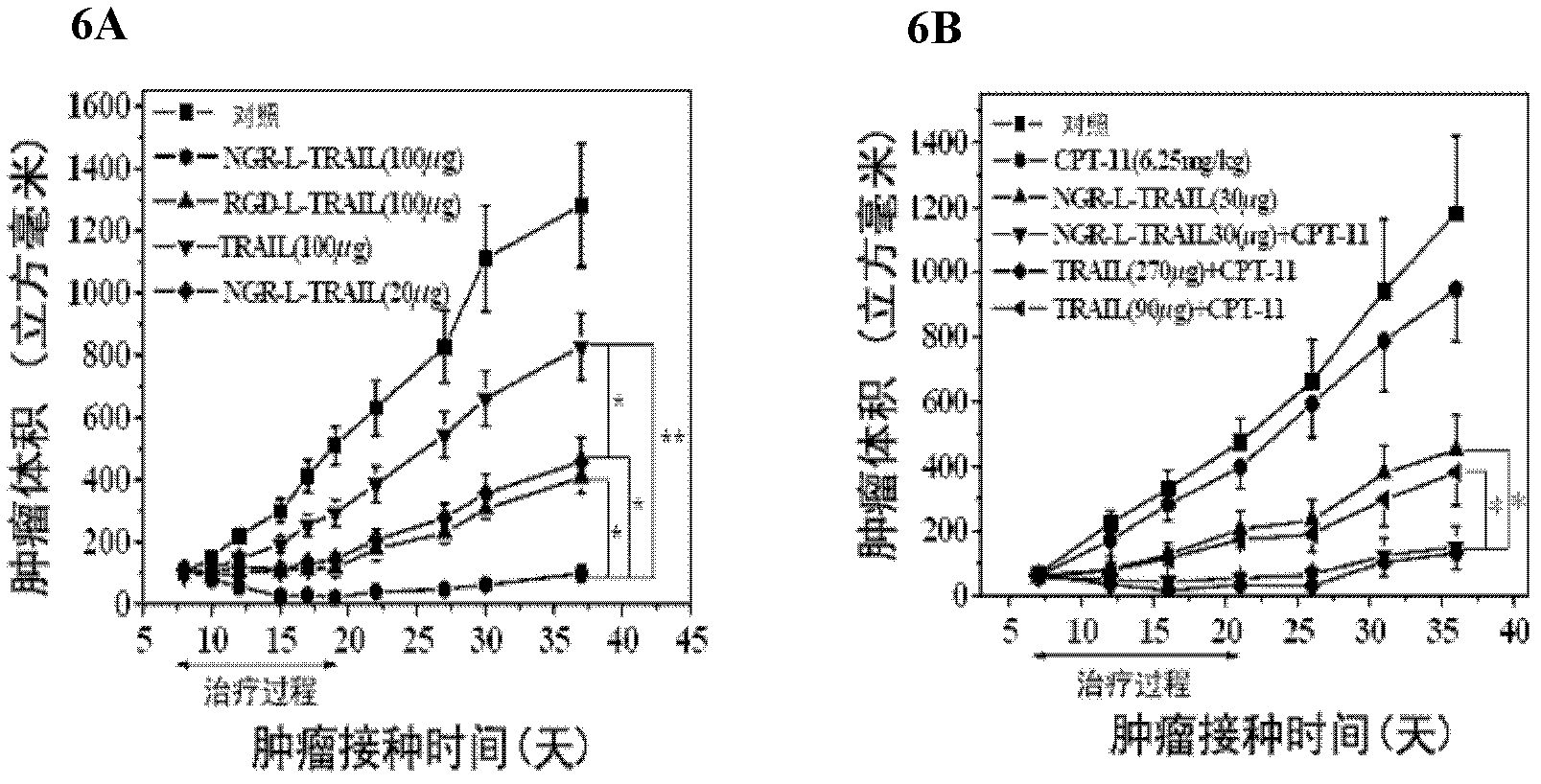
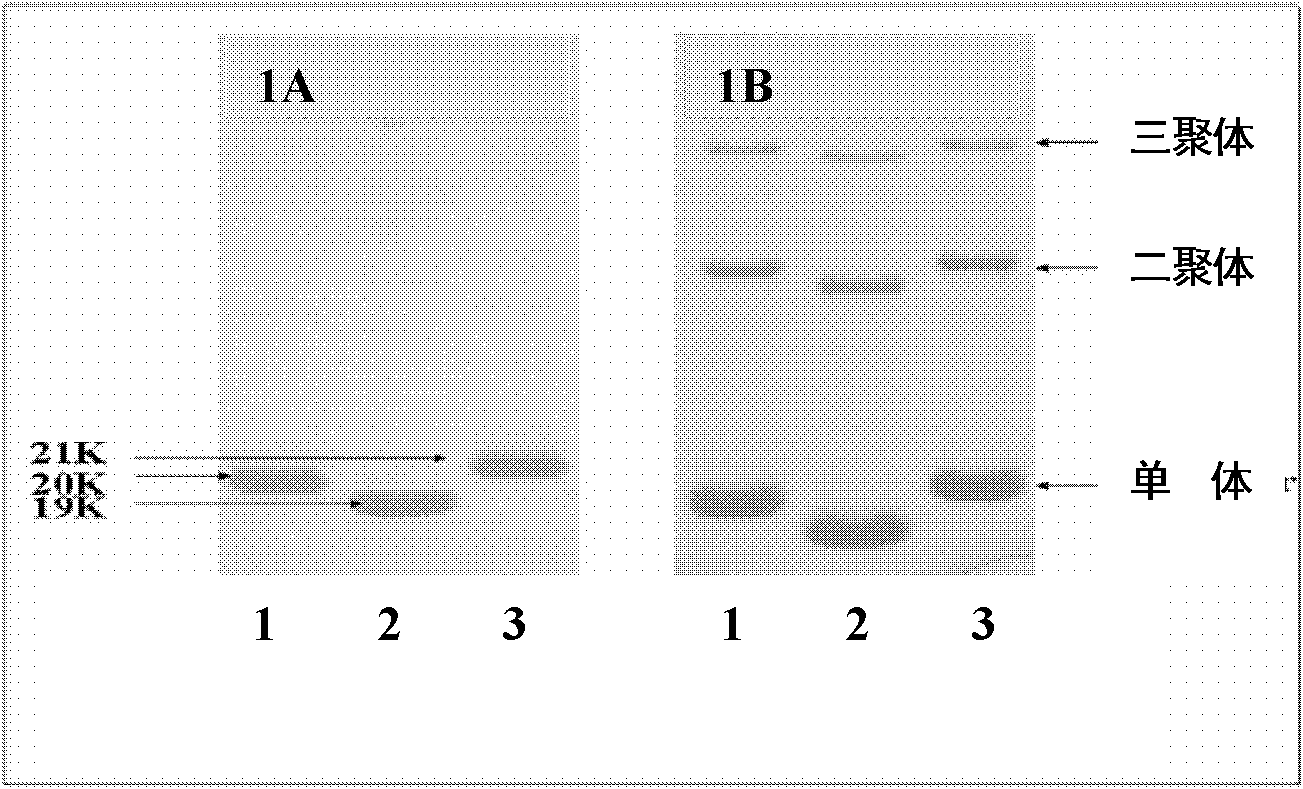
Tumor targeting tumor necrosis factor related apoptosis ligand variant and application thereof
A tumor necrosis factor, tumor-targeting technology, used in anti-tumor drugs, for targeting specific cell fusion, receptors/cell surface antigens/cell surface determinants, etc., can solve side effects, unpredictable immune results, etc. To achieve the effect of prolonging half-life, good pharmacokinetic effect, and good tumor tissue targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Preparation of tumor-targeting tumor necrosis factor-related apoptosis ligand variants
[0045] Using computer-aided structural simulation and molecular design, on the SGI computer workstation, using MSI company's molecular design software (InsightII, Discover and other modules), based on the crystal structure of tumor necrosis factor-related apoptosis ligands, the Add CD13 ligand short peptide to the N-terminal of the apoptosis ligand, carry out molecular modeling and molecular design, and determine the amino acid length of the connecting peptide.
[0046] On the basis of the above molecular design, we first chose the design scheme of linking peptide with 5 glycines, because computer simulation showed that the linking peptide of this scheme is shorter, and it has a great impact on the structure of tumor necrosis factor-related apoptosis ligand protein, CD13 ligand, etc. The combination of body and CD13 may have a greater impact. Most of the connecting peptides of othe...
Embodiment 2
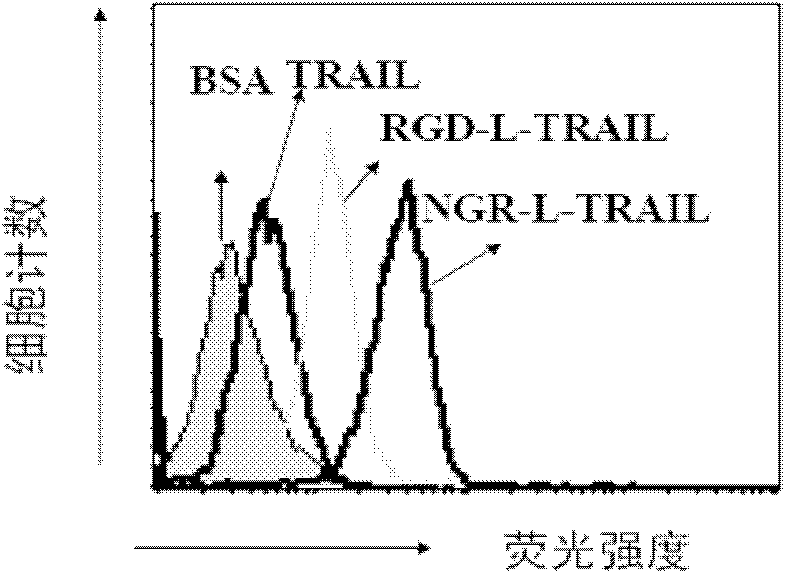
[0058] Binding assay of tumor necrosis factor-related apoptosis ligand and its tumor-targeting variants to endothelial cells
[0059] Tumor necrosis factor-related apoptosis ligands and their tumor-targeting variants NGR-L-TRAIL and RGD-L-TRAIL variants were labeled with fluorescein (Sigma) respectively, and the labeled proteins were passed through Sephadex-G25 molecular sieve Remove free fluorescein. Human foreskin microvascular endothelial cells were digested with trypsin, washed twice with ice-cold phosphate buffer containing 2% fetal bovine serum, then resuspended, added 1 microgram of labeled protein, and incubated at 4°C for 1 hour. Fluorescein-labeled bovine serum albumin was used as a control in the experiment. After the stained cells were washed three times, the binding capacity was analyzed by flow cytometry (Becton Dickinson Company).
[0060] We further evaluated the fluorescein-labeled tumor necrosis factor-related apoptosis ligand and its tumor-targeting varian...
Embodiment 3
[0062] Expression Analysis of CD13 and Integrin αVβ3, αVβ5
[0063] The expression abundance of CD13 or integrin on the cell surface was detected by flow cytometry analysis by indirect labeling method. The digested cells were washed twice with ice-cold phosphate buffer containing 2% fetal bovine serum, resuspended and coated with anti-human CD 13 monoclonal antibody (eBioscience) or anti-human αVβ3 integrin antibody MAB23C6 (eBioscience) on ice. , company) or anti-human αVβ5 integrin antibody MAB 1961 (Chemicon International Company) for 1 hour, and the negative control was purified isotype mouse immunoglobulin G (eBioscience Company). After the primary antibody-coated cells were washed twice, green fluorescein-conjugated goat anti-mouse immunoglobulin G1 (γ) (Caltag Laboratories) secondary antibody was added to label in the dark for 30 minutes. Then wash three times, wash and fix with phosphate buffer solution containing 4% formalin, and finally detect and analyze the abunda...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com