Recombinant human proinsulin transpeptidation method and application in recombinant human proinsulin downstream purification thereof
A technology for recombinant human insulin and insulin, which is applied in the biological field, can solve the problems of many by-products, complexity, and increasing the difficulty of insulin purification, and achieve the effects of improving production efficiency, shortening time, and solving the long time of transpeptide reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
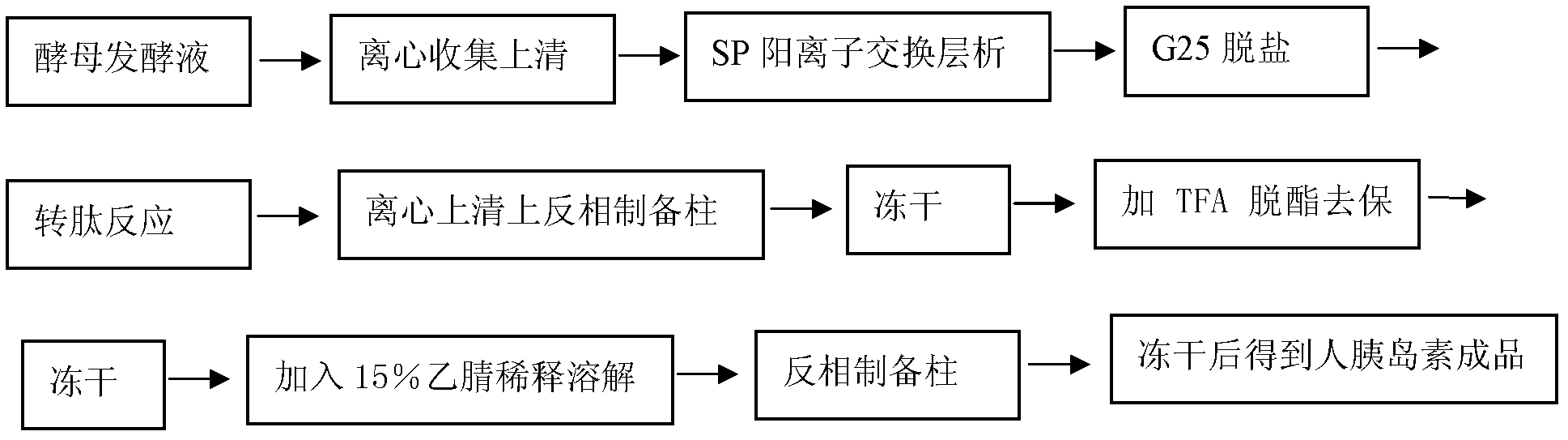
[0038] Embodiment 1 Purification of recombinant human insulin
[0039] Process steps:
[0040] A, Purification of proinsulin expressed by Pichia pastoris by SP cation exchange chromatography
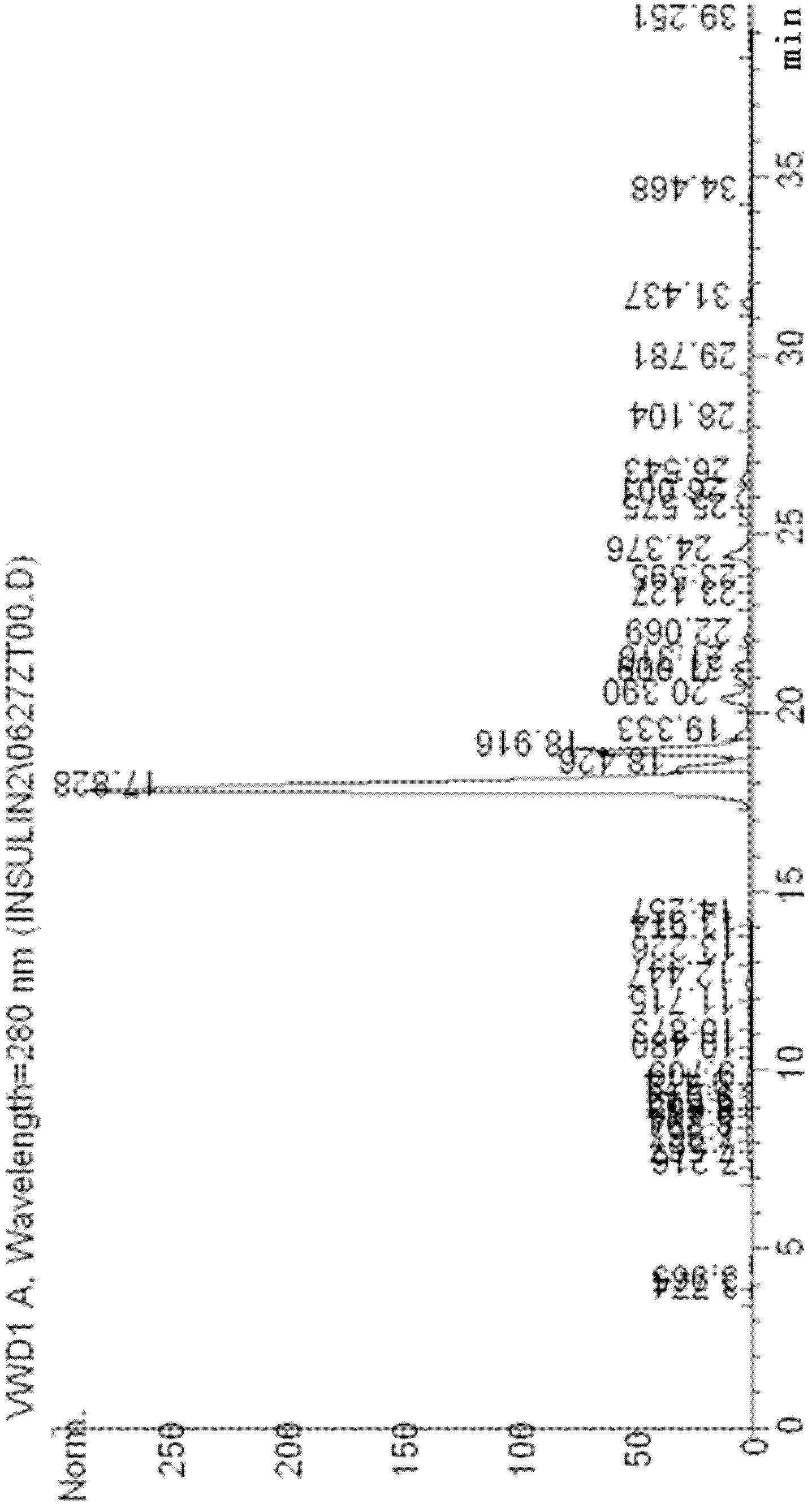
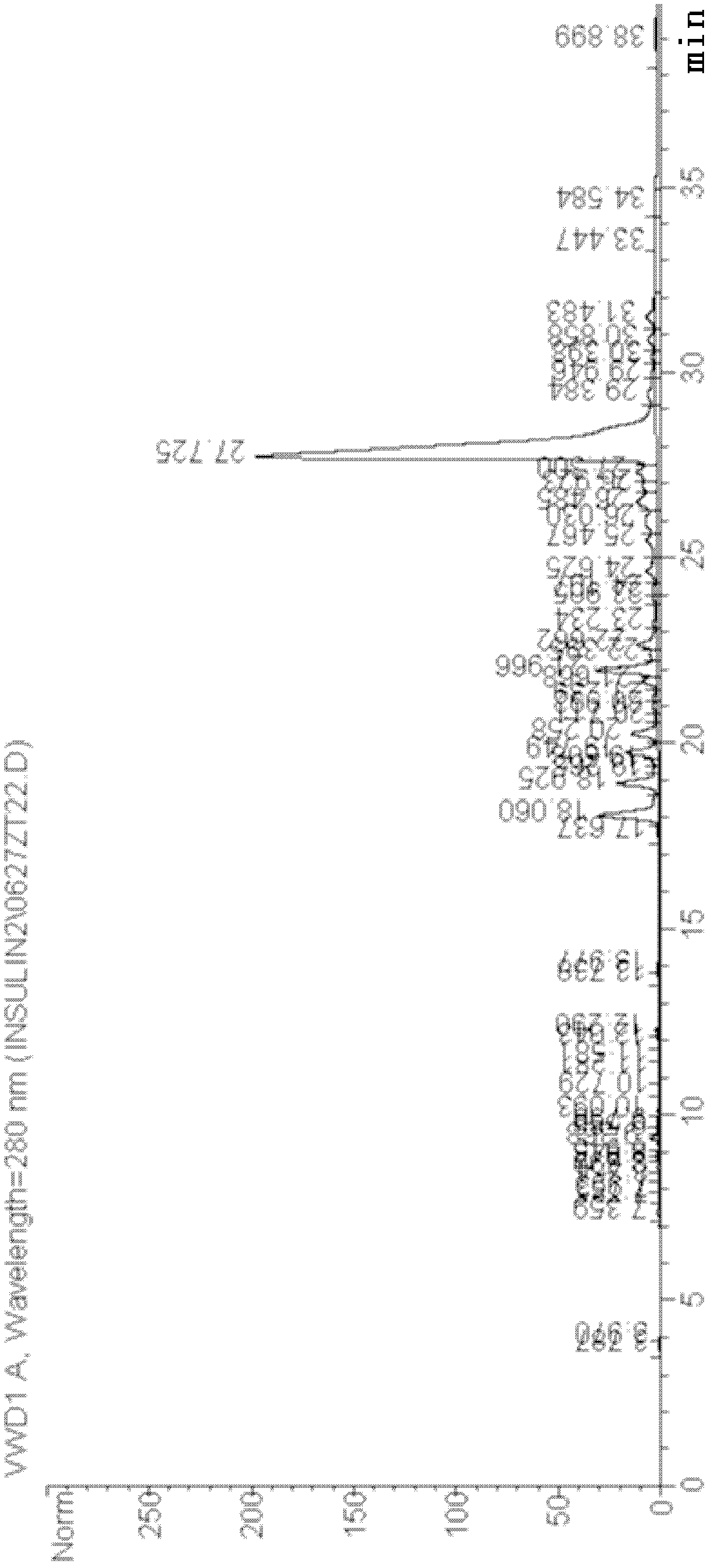
[0041] 4 liters of recombinant human proinsulin yeast fermentation broth (prepared according to the method described in Kjeldsen T, Pettersson AF, Hach M: Secretory expression and characterization of insulin in Pichia pastoris. Biotechnol Appl Biochem 1999, 29: 79-86), centrifuged at 7000RPM Collect the supernatant. SP Sepharose FF (purchased by GE) was packed with 200 ml of XK50 / 30 chromatographic column. First, the SP column was equilibrated for 5 column volumes with 25 mmol acetic acid-sodium acetate pH3.5 buffer solution. After the equilibrium, the pH of the centrifuged supernatant was adjusted to 3.5. After loading the sample, wash the column with the equilibration solution to the baseline, about 3 column volumes. Sodium dihydrogen phosphate / disodium hydrogen phosphate buffer sol...
Embodiment 2
[0073] Embodiment 2 Purification of recombinant human insulin
[0074] Process steps:
[0075] A, Purification of proinsulin expressed by Pichia pastoris by SP cation exchange chromatography
[0076] 4 liters of recombinant human proinsulin yeast fermentation broth (prepared according to the method described in Kjeldsen T, Pettersson AF, Hach M: Secretory expression and characterization of insulin in Pichia pastoris. Biotechnol Appl Biochem 1999, 29: 79-86), centrifuged at 7000RPM Collect the supernatant. SP Sepharose FF (purchased by GE) was packed with 200 ml of XK50 / 30 chromatographic column. First, the SP column was equilibrated for 5 column volumes with 25 mmol acetic acid-sodium acetate pH3.5 buffer solution. After the equilibrium, the pH of the centrifuged supernatant was adjusted to 3.5. After loading the sample, wash the column with the equilibration solution to the baseline, about 3 column volumes. Sodium dihydrogen phosphate / disodium hydrogen phosphate buffer sol...
Embodiment 3
[0096] Embodiment 3 Purification of recombinant human insulin
[0097] Process steps:
[0098] A, Purification of proinsulin expressed by Pichia pastoris by SP cation exchange chromatography
[0099] 4 liters of recombinant human proinsulin yeast fermentation broth (prepared according to the method described in Kjeldsen T, Pettersson AF, Hach M: Secretory expression and characterization of insulin in Pichia pastoris. Biotechnol Appl Biochem 1999, 29: 79-86), centrifuged at 7000RPM Collect the supernatant. SP Sepharose FF (purchased by GE) was packed with 200 ml of XK50 / 30 chromatographic column. First, the SP column was equilibrated for 5 column volumes with 25 mmol acetic acid-sodium acetate pH3.5 buffer solution. After the equilibrium, the pH of the centrifuged supernatant was adjusted to 3.5. After loading the sample, wash the column with the equilibration solution to the baseline, about 3 column volumes. Sodium dihydrogen phosphate / disodium hydrogen phosphate buffer solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com