FGFR single-stranded antibody fusion protein and application thereof in preparing targeting tumor cells medicines
A fusion protein and single-chain antibody technology, applied in the fields of genetic engineering and biopharmaceuticals, can solve the problem of not being able to fundamentally eradicate tumors, and achieve the effects of increasing protein expression and soluble protein content.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 PCR synthesis of FGFR3 ScFV-basic polypeptide fusion gene
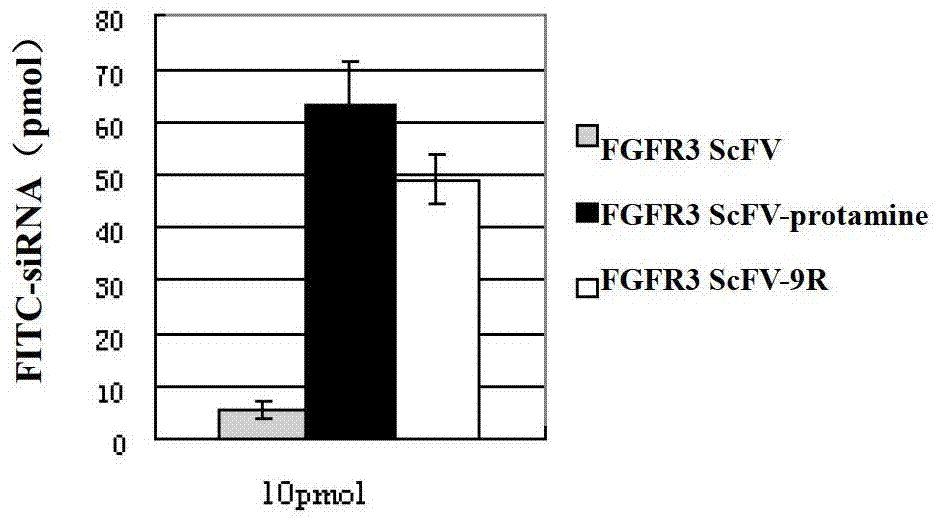
[0042] According to the gene sequence encoding FGFR3 single-chain antibody reported in the literature (Martínez-Torrecuadrada J et al., 2005), Shanghai Jierui Bioengineering Co., Ltd. was entrusted to synthesize the fusion gene of FGFR3ScFV and basic polypeptide. Among them, the FGFR3 single-chain antibody-fish The nucleotide sequences of the protamine fusion gene and the FGFR3 single-chain antibody-polyarginine 9R fusion gene are Seq ID No4 and No. 6, respectively.
Embodiment 2
[0043] Example 2 Construction of pET-20b-Sumo-FGFR3 ScFV-basic polypeptide expression vector
[0044] Refer to the vector construction method described in ZL200810102542.4. In order to fuse the synthetic FGFR3 ScFV-basic polypeptide fusion gene with the nucleotide sequence encoding the Sumo molecular chaperone, the synthetic primer P1 was designed according to the N-terminal of the Sumo sequence, and the primer P2 was designed according to the Sumo C-terminal and the ScFV N-terminal. P2 contained 20bp and ScFV-basic polypeptide gene complementary sequence. Using P1 and P2 as primers (see Table 1 for primer sequences), the Sumo-S sequence was synthesized by PCR.
[0045] PCR reaction system one:
[0046]
[0047] Reaction program: denaturation at 94°C for 5 min; denaturation at 94°C for 1 min; annealing at 55°C for 0.5 min; extension at 72°C for 0.5 min, 30 cycles, and finally extension at 72°C for 10 min. After no non-specific bands were detected by 2% agarose gel electr...
Embodiment 3
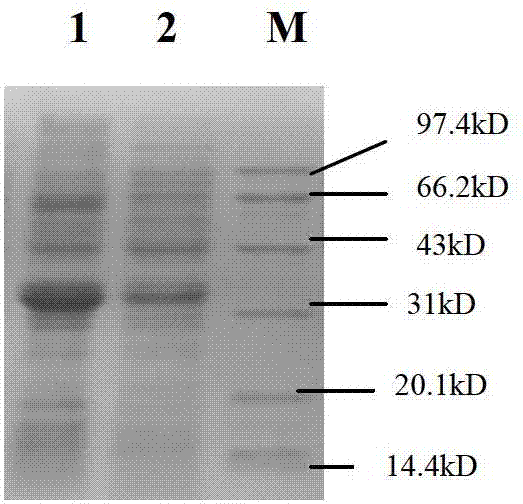
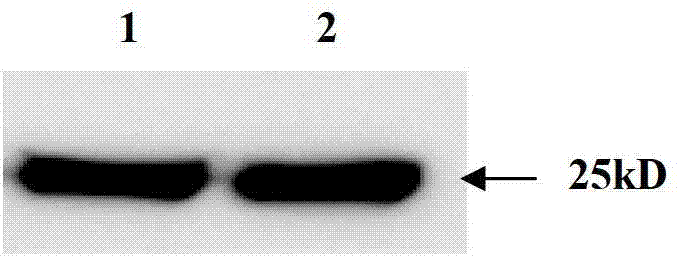
[0056] Example 3 Obtaining of FGFR3 ScFV-Basic Polypeptide Fusion Protein
[0057] 1. Induced expression
[0058] The correctly sequenced pET-20b-Sumo-FGFR3 ScFV-basic polypeptide plasmid was transformed into Escherichia coli BL21 (DE3) competent cells, and positive transformants were screened on an ampicillin resistance plate. A single colony of the screened positive recombinant bacteria BL21(DE3) / pET-20b-Sumo-ScFV-basic polypeptide was inoculated into LB medium and cultured with shaking at 37°C for 16h. Then it was transferred to fresh LB medium at a ratio of 5%, and the expression conditions such as temperature, IPTG concentration and induction time were optimized. The results showed that when the OD value was 0.6, the bacteria were induced by 1mM IPTG at 37°C for 3h, ultrasonic (100w, ultrasonic 2s, interval 2s) broke the bacteria, and the soluble expressed target protein could account for more than 90% of the total bacterial target protein.
[0059] 2. Protein Purificat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com