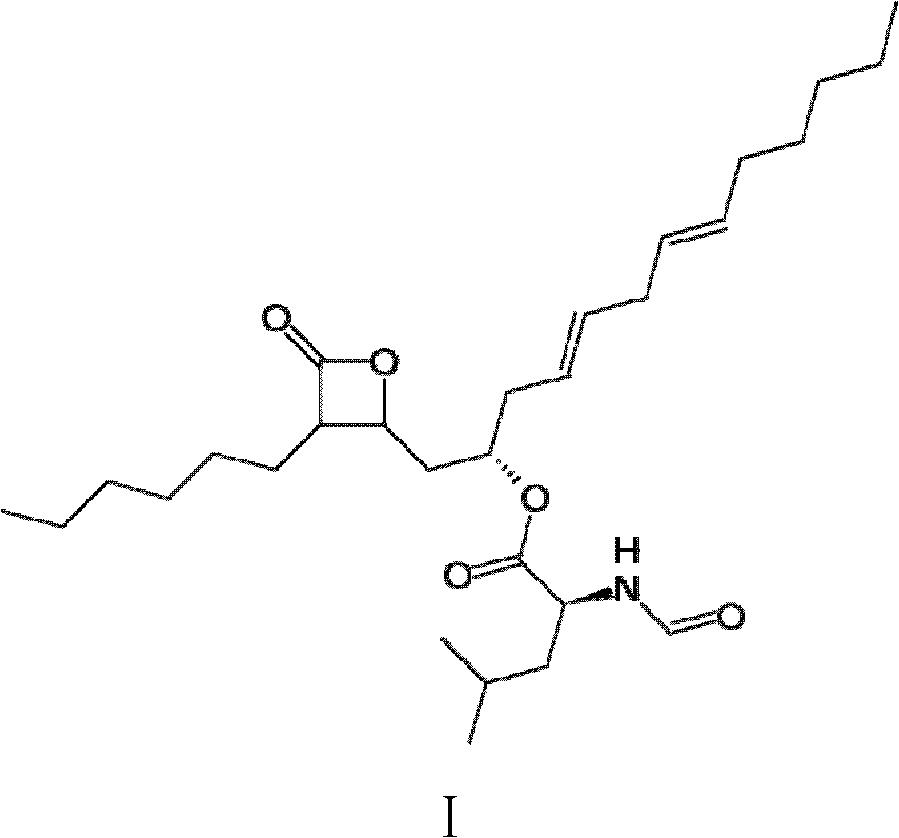
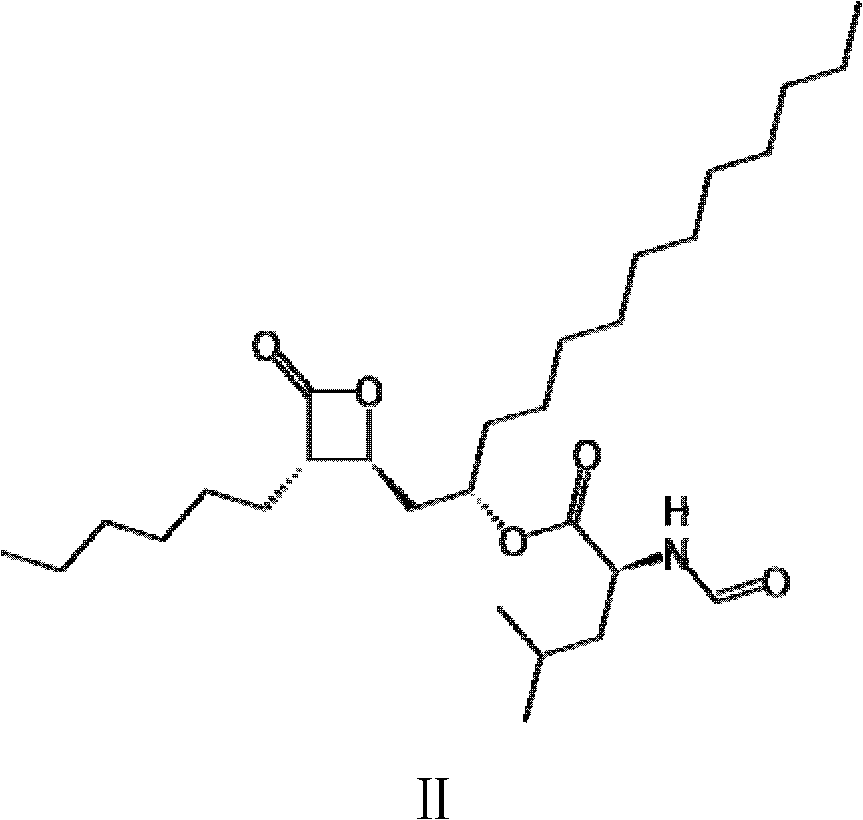
Method for production of lipstatin through fermentation
A technology of lipox and statin, applied in the field of biopharmaceuticals, to achieve the effects of simple production and operation, low price and wide sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] 1. Preparation of methyl silicone oil feeding solution:
[0050] Accurately weigh 3 g (w / v) of methyl silicone oil, add 100 ml of purified water, stir, sterilize by high-pressure steam at 121°C for 15 minutes, and cool to obtain a sterile oxygen carrier feed solution. Prepare 5 parts of the same methyl silicone oil feeding solution.
[0051] 2. Ferment and cultivate Streptomyces toxin as follows:
[0052] The spores were inserted into 150ml seed culture medium, and the proportion of the culture medium was (w / v): 1.5% soybean powder, 2.5% glycerin, 1% soybean oil, and the balance was water; pH 7.0; sterilized at 121° C. for 30 minutes. The shaker rotates at 250 rpm, cultures at 28° C. for 20 hours, and transfers to 3 L of secondary seed medium, and the transfer amount is 5%.
[0053] The ratio of the secondary seed medium is (w / v): 2% soybean powder, 3% glycerin, 1.5% soybean oil, 0.1% potassium dihydrogen phosphate, 0.1% magnesium sulfate, and the balance is water; pH...
Embodiment 2
[0058] 1. Preparation of hemoglobin feed solution:
[0059] Accurately weigh 0.3 g of hemoglobin, add 50 ml of sterile distilled water to dissolve, and filter to sterilize with a microporous membrane with a pore size of 0.25 μm.
[0060] Prepare 5 parts of the above hemoglobin solution for later use.
[0061] 2. Ferment and cultivate Streptomyces toxin as follows:
[0062] The spores were inserted into 150ml seed culture medium, and the proportion of the culture medium was (w / v): 1.5% soybean powder, 2.5% glycerin, 1% soybean oil, and the balance was water; pH 7.0; sterilized at 121° C. for 30 minutes. The shaker rotates at 250 rpm, cultures at 28° C. for 20 hours, and transfers to 3 L of secondary seed medium, and the transfer amount is 5%.
[0063] The ratio of the secondary seed medium is (w / v): 2% soybean powder, 3% glycerin, 1.5% soybean oil, 0.1% potassium dihydrogen phosphate, 0.1% magnesium sulfate, and the balance is water; pH 7.0; 121 °C for 25 minutes. Stir at 3...
Embodiment 3
[0068] 1. Preparation of carbon tetrafluoride feeding solution:
[0069] Accurately weigh 5 g of carbon tetrafluoride, add 100 ml of purified water, stir, sterilize by high-pressure steam at 121° C. for 15 minutes, and cool to obtain a sterile oxygen carrier feeding solution. Prepare 5 parts of the same carbon tetrafluoride feeding solution.
[0070] 2. Ferment and cultivate Streptomyces toxin as follows:
[0071] The spores were inserted into 150ml seed culture medium, and the proportion of the culture medium was (w / v): 1.5% soybean powder, 2.5% glycerin, 1% soybean oil, and the balance was water; pH 7.0; sterilized at 121° C. for 30 minutes. The shaker rotates at 250 rpm, cultures at 28° C. for 20 hours, and transfers to 3 L of secondary seed medium, and the transfer amount is 5%.
[0072] The ratio of the secondary seed medium is (w / v): 2% soybean powder, 3% glycerin, 1.5% soybean oil, 0.1% potassium dihydrogen phosphate, 0.1% magnesium sulfate, and the balance is water; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com