Antidotal capsule of Tibetan medicine compound and quality detection method of preparation of antidotal capsule
A quality inspection method and the technology of Jiedu capsules are applied in the direction of material analysis, measuring devices, instruments, etc. by observing the impact on chemical indicators, which can solve problems such as harm to human health, safety of preparations, acute iron poisoning, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0278] Embodiment 1: Jiedu capsule quality detection method
[0279] Jiedu Capsule includes bile acid TLC identification, gallic acid content measurement items (or not including these measurement items), and also includes the following measurement items:
[0280] A. Determination of Nuxychon content:
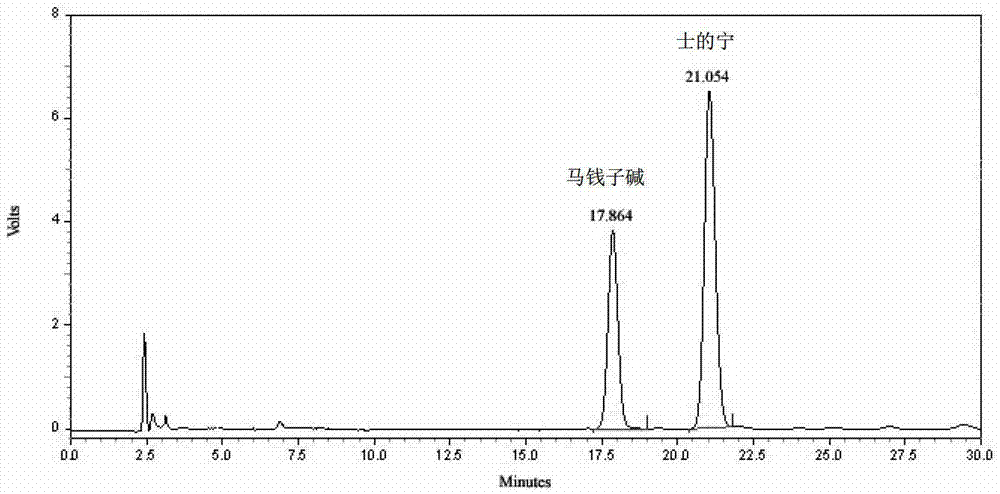
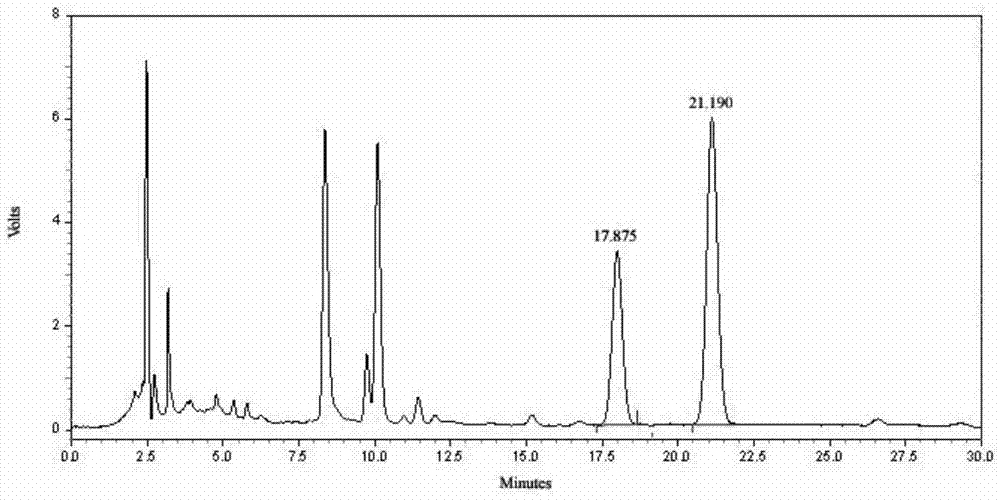
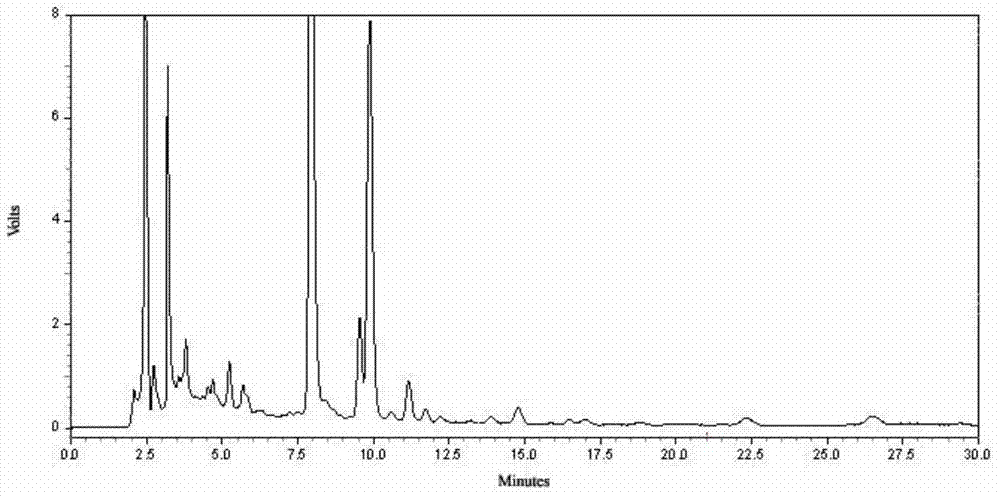
[0281] Determine according to high performance liquid chromatography (Appendix VID of "Chinese Pharmacopoeia" 2010 edition);
[0282] Chromatographic conditions and system suitability test: use octadecylsilane bonded silica gel as filler; use acetonitrile-0.01mol / l sodium heptanesulfonate and 0.02mol / l potassium dihydrogen phosphate at a ratio of 20:80 by volume Equal volume mixed solution (adjust the pH value to 2.8 with 10% phosphoric acid by volume ratio) as the mobile phase; the detection wavelength is 260nm; the number of theoretical plates should not be less than 5000 based on the strychnine peak;
[0283] Preparation of reference substance solution: Take 6 mg of strychn...
Embodiment 2
[0304] Embodiment 2: Method for detecting the quality of detoxifying granules
[0305] Detoxification granules include bile acid thin-layer identification, gallic acid content measurement items (or not including these measurement items), and also include the following measurement items:
[0306] A. Determination of Nuxychon content:
[0307] Determine according to high performance liquid chromatography (Appendix VID of "Chinese Pharmacopoeia" 2010 edition);
[0308] Chromatographic conditions and system suitability test: use octadecylsilane bonded silica gel as filler; use acetonitrile-0.01mol / l sodium heptanesulfonate and 0.02mol / l potassium dihydrogen phosphate at a ratio of 20:80 by volume Equal volume mixed solution (adjust the pH value to 2.8 with 10% phosphoric acid by volume ratio) as the mobile phase; the detection wavelength is 260nm; the number of theoretical plates should not be less than 5000 based on the strychnine peak;
[0309] Preparation of reference substan...
Embodiment 3
[0331] Embodiment 3: Jiedu pill quality detection method
[0332] Jiedu pills include bile acid thin-layer identification, gallic acid content measurement items (or may not include these measurement items), and also include the following measurement items:
[0333] A. Determination of Nuxychon content:
[0334] Determine according to high performance liquid chromatography (Appendix VID of "Chinese Pharmacopoeia" 2010 edition);
[0335] Chromatographic conditions and system suitability test: use octadecylsilane bonded silica gel as filler; use acetonitrile-0.01mol / l sodium heptanesulfonate and 0.02mol / l potassium dihydrogen phosphate at a ratio of 20:80 by volume Equal volume mixed solution (adjust the pH value to 2.8 with 10% phosphoric acid by volume ratio) as the mobile phase; the detection wavelength is 260nm; the number of theoretical plates should not be less than 5000 based on the strychnine peak;
[0336] Preparation of reference substance solution: Take 6 mg of stryc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com