Electrolyte of lithium ion battery
A lithium-ion battery and electrolyte technology, applied in secondary batteries, circuits, electrical components, etc., can solve the problems of low oxidation resistance and large impedance, and achieve good thermal stability and great application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1 : In a glove box filled with argon, measure 1 mL SL and 1 mL DMS respectively, mix thoroughly, add 0.2713 g of electrolyte lithium salt LiBOB, stir until the lithium salt is completely dissolved, the ratio is: 0.7 mol / L LiBOB-SL / DMS.
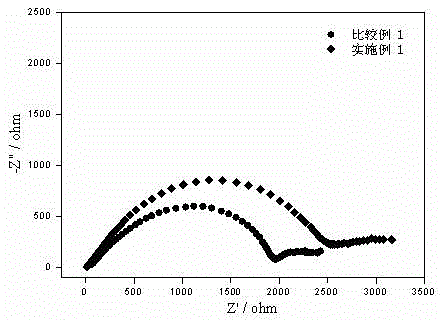
[0016] figure 1 It is the impedance comparison diagram of the negative electrodes at room temperature of Example 1 and Comparative Example 1 after the first cycle, and the negative electrode material of the assembled battery is mesocarbon microspheres (MCMB). It can be seen that the impedance of the SEI film formed by the first cycle at room temperature in Example 1 is significantly lower than that of Comparative Example 1, indicating that the internal resistance of the battery assembled with the new electrolyte will be lower than that of LiPF 6 Battery.
Embodiment 2
[0017] Example 2 : In a glove box filled with argon, measure 1 mL SL and 1 mL DEC respectively, mix thoroughly, add 0.2713 g of electrolyte lithium salt LiBOB, stir until the lithium salt is completely dissolved, the ratio is: 0.7 mol / L LiBOB-SL / DEC.
Embodiment 3
[0018] Example 3 : In a glove box filled with argon, measure 1 mL SL, 1 mL GBL, and 1 mL DMC respectively. After mixing well, add 0.5791 g of electrolyte lithium salt LiBOB, and stir until the lithium salt is completely dissolved. The ratio is: 1.0 mol / L LiBOB-SL / GBL / DMC.
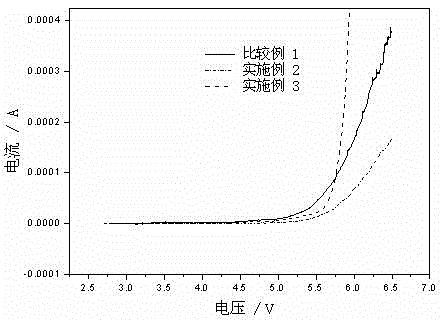
[0019] figure 2 It is the oxidation potential graph of embodiment 2, 3 and comparative example 1. It can be seen that the oxidation potential of the example is significantly higher than that of the comparative example.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com