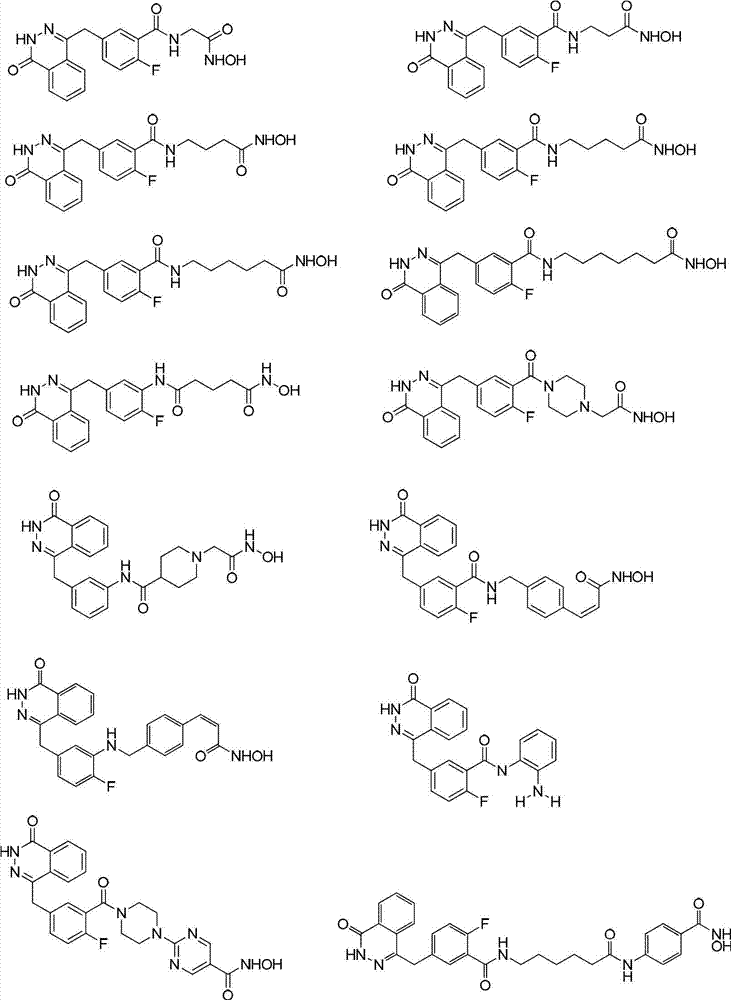
Phthalazinone derivatives and application thereof
A technology of use, medicine, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
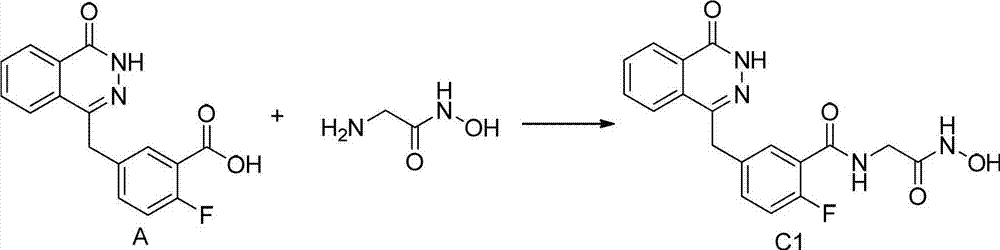
[0053] Example 1: 2-fluoro-[N-(2-hydroxylamine-2-oxoethyl)]-5-(4-oxo-3,4-dihydrophthalazine-1-methyl)benzidine Preparation of amides (C1)
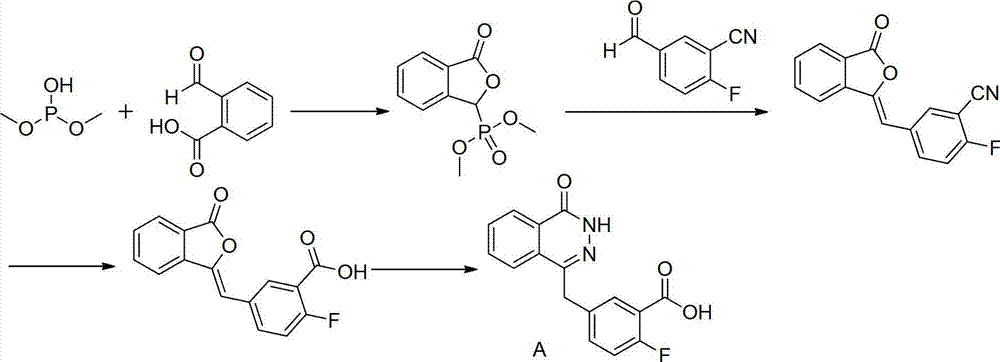
[0054] 1) Preparation of intermediate 2-fluoro-5-(4-oxo-3,4-dihydrophthalazine-1-methyl)benzoic acid (A)
[0055]
[0056] People such as Menear, KeithA reported a kind of intermediate 2-fluoro-5-(4-oxo-3,4-dihydrophthalazine-1-methyl) benzoic acid (A) [Journal of Medicinal Chemistry, 51( 20), 6581-6591; 2008], the compound A used in this patent is prepared according to this method.
[0057] 2) 2-fluoro-N-(2-hydroxyamino-2-oxoethyl)-5-(4-oxo-3,4-dihydrophthalazine-1-methyl)benzamide (C1) preparation of
[0058]
[0059] 2-Fluoro-5-(4-oxo-3,4-dihydrophthalazine-1-methyl)benzoic acid (A) (450mg, 1.51mmol), 2-amino-N-hydroxy-acetamide (136.1mg, 1.51mmol), HATU (689mg, 1.81mmol), DIPEA (584mg, 4.53mmol) were added to DMF 10ML, under nitrogen protection, stirred, reacted at room temperature for 12h, concentrated to dryness, added wate...
Embodiment 2
[0061] Example 2: 2-fluoro-[N-(3-hydroxyamino-3-oxopropyl)]-5-(4-oxo-3,4-dihydrophthalazine-1-methyl)benzidine Preparation of amides (C2)
[0062]
[0063] Compound 2 (197 mg, 34%) was prepared in a similar manner to compound 1. HPLC: 97.03%, MS: [M+H] + =385.0; [M+Na] + =406.8;[M-H] - =383.0.
[0064] 1 H-NMR (400M, DMSO-d 6 )δ12.59(s,1H),10.46(s,1H),8.75(s,1H),8.29(m,2H),7.96(d,1H),7.89(t,1H),7.83(t,1H ), 7.60 (d, 1H), 7.46 (m, 1H), 7.21 (t, 1H), 4.33 (s, 2H), 3.43 (td, 2H), 2.23 (t, 2H) ppm.
Embodiment 3
[0065] Example 3: 2-fluoro-[N-(4-hydroxylamine-4-oxo-n-butyl)]-5-(4-oxo-3,4-dihydrophthalazine-1-methyl)benzene Formamide (C3) Preparation
[0066]
[0067] Compound 3 (240 mg, 40%) was prepared in a similar manner to compound 1. HPLC: 98.7%, MS: [M-H] - =396.9.
[0068] 1 H-NMR (400M, DMSO-d 6 )δ12.60(s,1H),10.38(s,1H),8.71(s,1H),8.27(m,2H),7.97(d,1H),7.89(t,1H),7.83(t,1H ),7.57(d,1H),7.44(m,1H),7.20(t,1H),4.33(s,2H),3.20(td,2H),1.98(t,2H),1.71(td,2H) ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com