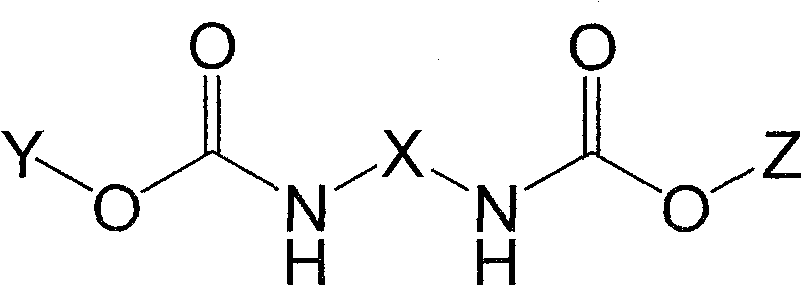
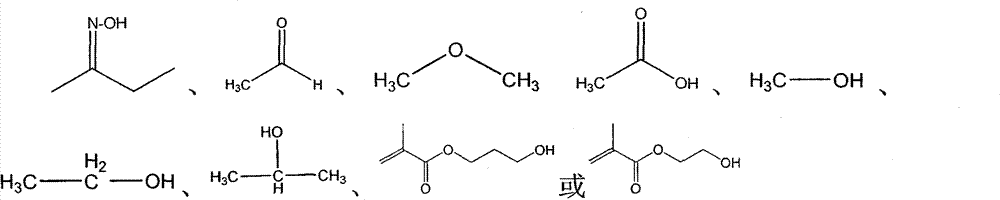
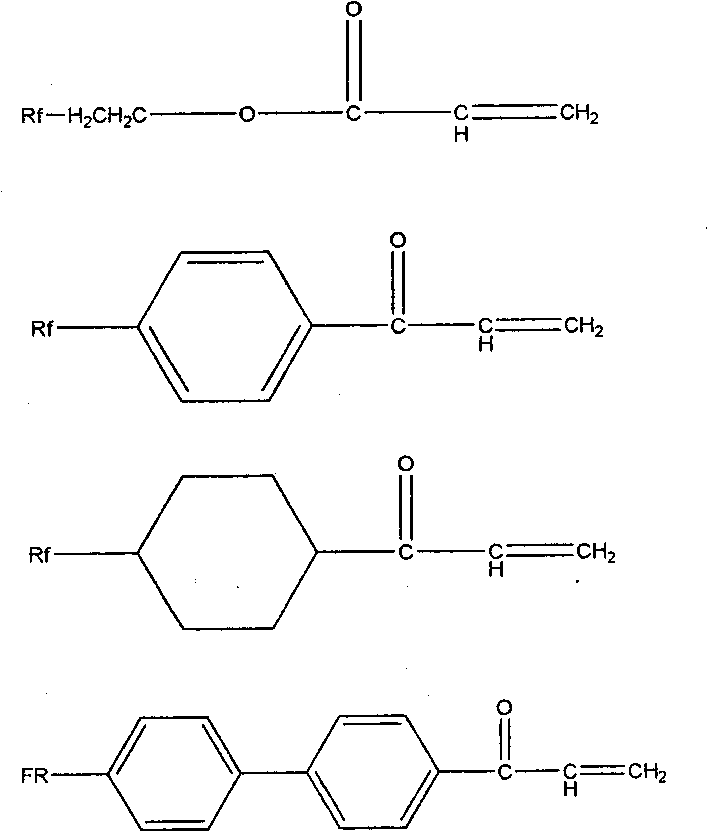
Fluorine water-repellent and oil-repellent agent
A water-repellent, oil-repellent, fluorine-based technology, applied in the preparation of organic compounds, carbamic acid derivatives, organic chemistry, etc., can solve problems such as monomer residues and acrylic hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0109] Synthesis Example 1: Fluorine-containing monomer with urethane structure (D-V1)
[0110] Place 100 grams of IPDI monomer and 0.03 grams of catalyst ES 100Ag catalyst in a three-neck round bottom bottle equipped with a mechanical stirrer, and add 55.6 grams of HEMA (2-hydroxyethyl methacrylate) into the round bottom bottle by dropwise addition, with full temperature control Below 40°C and react for 3 hours. In the second step, add 163 grams of dodecafluoroheptanol (2,2,3,3,4,4,5,5,6,6,7,7-dodecafluorooctan-1-ol) into the circle dropwise In the bottom bottle, the temperature was controlled below 40°C throughout the whole process and the reaction was carried out for 4 hours. After the reaction is completed, 318.6 grams of transparent and colorless mucus can be obtained.
[0111]
Synthetic example 2
[0112] Synthesis Example 2: Fluorine-containing monomer with urethane structure (D-V2)
[0113] Place 100 grams of IPDI monomer and 0.03 grams of catalyst ES 100Ag catalyst in a three-neck round bottom bottle equipped with a mechanical stirrer, and add 55.6 grams of 2-hydroxypropyl methacrylate into the round bottom bottle by dropwise, and the temperature control throughout the process is below 40 °C and reacted for 3 hours. In the second step, 156.6 grams of dodecafluoroheptanol (2,2,3,3,4,4,5,5,6,6,7,7-dodecafluoroheptan-1-ol) was added dropwise into the circle In the bottom bottle, the temperature was controlled below 40°C throughout the whole process and the reaction was carried out for 4 hours. After the reaction is completed, 312.2 grams of transparent and colorless mucus can be obtained.
[0114]
Synthetic example 3
[0115] Synthesis Example 3: Fluorine-containing monomer with urethane structure (D-V3)
[0116] Place 100 grams of IPDI monomer and 0.03 grams of catalyst ES 100Ag catalyst, 149.4 grams of dodecafluoroheptanol (2,2,3,3,4,4,5,5) in a three-necked round bottom bottle equipped with a mechanical stirrer , 6,6,7,7-dodecafluorooctan-1-ol) was added dropwise into the round bottom bottle, the temperature was controlled below 40°C throughout the whole process and reacted for 3 hours. In the second step, 41.2 grams of methyl ethyl ketone oxime (MEKO) was added dropwise into the round bottom bottle, and the temperature was controlled below 40°C throughout the process and reacted for 4 hours. After the reaction is completed, 290.6 grams of transparent and colorless mucus can be obtained.
[0117]
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com