Acid-sensitive degradable polymer vesicle and preparation and application thereof
A technology for degrading polymers and polymers, applied in capsule delivery, microcapsules, nanocapsules, etc., can solve the problem of drug resistance of cancer cells and achieve high mechanical strength, good colloidal stability, and low chemical penetration sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
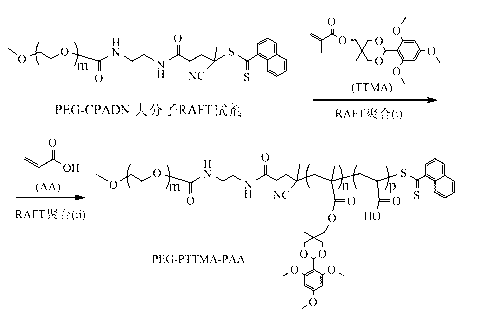
[0035] Example 1, Synthesis of triblock polymer PEG-PTTMA-PAA (1.5k) by sequential RAFT method
[0036] Under a nitrogen atmosphere, dissolve 0.16 g of TTMA (0.44 mmol) and 0.05 g of PEG-CPADN (0.01 mmol) in 2 mL of DMF, then add 0.23 mg of AIBN (0.0014 mmol), and the reaction system is aerated for 30 minutes , and then placed in an oil bath at 65°C for two days. After the reaction was complete, a drop of the reaction mixture was removed to determine the conversion of the monomer. The rest was used to continue the reaction. Add 0.01 g (0.14 mmol) of the second monomer acrylic acid and 0.23 mg of AIBN (0.0014 mmol) to the reaction system, and continue the reaction at 65 °C for two days. After the reaction, the mixture was precipitated with anhydrous ether and dried in vacuum for one day to obtain a pink solid with a yield of 73.2%. NMR results show that the molecular weight of PAA is 1500, and its structure is marked as PEG-PTTMA-PAA (1.5k). Proton NMR spectrum (400 MHz, CDC...
Embodiment 2
[0037] Example 2, Synthesis of triblock polymer PEG-PTTMA-PAA (2.1k) by RAFT method
[0038] Under a nitrogen atmosphere, dissolve 0.16 g of TTMA (0.44 mmol) and 0.05 g of PEG-CPADN (0.01 mmol) in 2 mL of DMF, then add 0.23 mg of AIBN (0.0014 mmol), and the reaction system is aerated for 30 minutes , and then placed in an oil bath at 65°C for two days. After the reaction was complete, a drop of the reaction mixture was removed to determine the conversion of the monomer. The rest was used to continue the reaction, adding 0.02 g (0.28 mmol) of the second monomer acrylic acid and 0.23 mg of AIBN (0.0014 mmol) to the reaction system, and continued the reaction at 65°C for two days. After the reaction, the mixture was precipitated with anhydrous ether and dried in vacuum for one day to obtain a pink solid with a yield of 79.0%. NMR results show that the molecular weight of PAA is 2100, and its structure is marked as PEG-PTTMA-PAA (2.1k).
Embodiment 3
[0039] Example 3, Synthesis of triblock polymer PEG-PTTMA-PAA (2.7k) by RAFT method
[0040] Under a nitrogen atmosphere, dissolve 0.16 g of TTMA (0.44 mmol) and 0.05 g of PEG-CPADN (0.01 mmol) in 2 mL of DMF, then add 0.23 mg of AIBN (0.0014 mmol), and the reaction system is aerated for 30 minutes , and then placed in an oil bath at 65°C for two days. After the reaction was complete, a drop of the reaction mixture was removed to determine the conversion of the monomer. The rest was used to continue the reaction. Add 0.03 g (0.42 mmol) of the second monomer acrylic acid and 0.23 mg of AIBN (0.0014 mmol) to the reaction system, and continue the reaction at 65 °C for two days. After the reaction, the mixture was precipitated with anhydrous ether and dried in vacuum for one day to obtain a pink solid with a yield of 81.7%. NMR results show that the molecular weight of PAA is 2700, and its structure is marked as PEG-PTTMA-PAA (2.7k).
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com