Application of 4-hydroxy salicylanilide to preparation of medicament for preventing and treating hepatitis B
A technology of hydroxysalicylanilide and hepatitis B, applied in the field of application of 4-hydroxysalicylanilide in the preparation of drugs for the prevention and treatment of hepatitis B, which can solve the problems of lowering blood cholesterol and achieve the effect of high killing activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
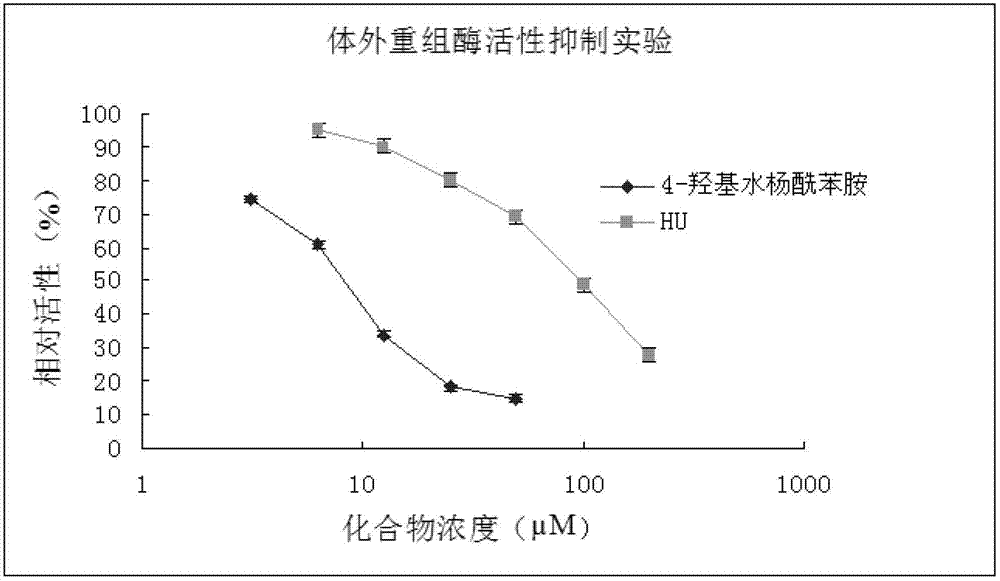
[0027] Embodiment 1: the recombinant RR enzyme inhibitory activity of medicine
[0028] 1. Experimental materials
[0029] (1) Main reagents: hydroxyurea (Hydroxyurea, HU, Sigma), 4-hydroxysalicylanilide (Shanghai Titan Chemical Co., Ltd.).
[0030] (2) Main instruments: HPLC (Shimadzu LC-10ATVP), liquid scintillation counter (Backman LS6500).
[0031] 2. Experimental method
[0032] (1) Sample preparation for recombinant RR enzyme activity assay
[0033] 1) Preparation of reaction samples: Escherichia coli expressed in vitro the purified recombinant human RR enzyme large subunit M1 (see SEQ ID NO.1 for the sequence) and small subunit M2 protein (see SEQ ID NO.2 for the sequence) (the amount of protein used was RR enzyme Activity reaches 65~95%), add double distilled water to adjust the volume to 80μl, add 10μl of different concentrations of test compounds; mix well, and incubate at room temperature for 30~60min;
[0034] 2) Add reaction buffer to each tube to a volume of ...
Embodiment 2
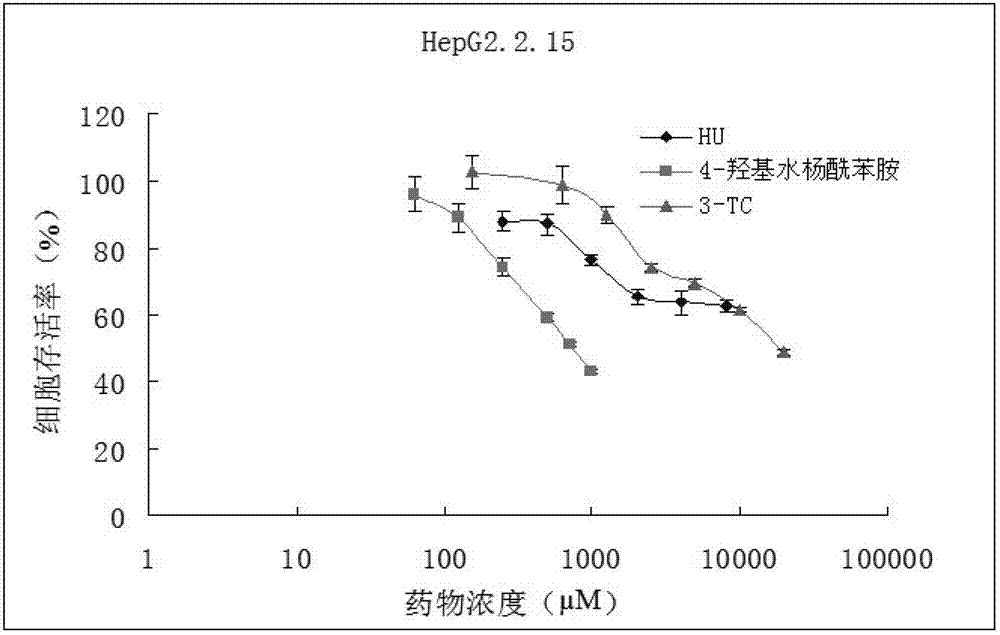
[0043] Embodiment 2: the determination of drug test dosage
[0044] 1. Experimental materials
[0045] (1) Cell line: HepG2.2.15 cells (stable HBV gene transfection, which can stably replicate and express HBV genome) (gifted by the Institute of Infectious Diseases, Zhejiang University).
[0046] (2) Main reagents: DMEM medium (Gibco, USA), fetal bovine serum (Gibco, USA), hydroxyurea (Hydroxyurea, HU, Sigma), 4-hydroxysalicylanilide (Shanghai Titan Chemical Co., Ltd.), La Mivudine (Lamivudine, 3-TC, Tokyo Rensei Industry Co., Ltd., Japan); Cell Counting Kit-8 kit (CCK8, Tongjin Chemical Research Institute, Japan Co., Ltd.).
[0047] (3) Main instruments: carbon dioxide incubator (Thermo Forma, USA), automatic microplate reader (Bio-TEK, Elx800).
[0048] 2. Experimental method
[0049] (1) Cell culture:
[0050] Cells were cultured in DMEM high-glucose medium (DMEM medium supplemented with 10% fetal bovine serum, pH 7.2), added 400 μg / ml G418, 2 mmol / L glutamine, placed in...
Embodiment 3
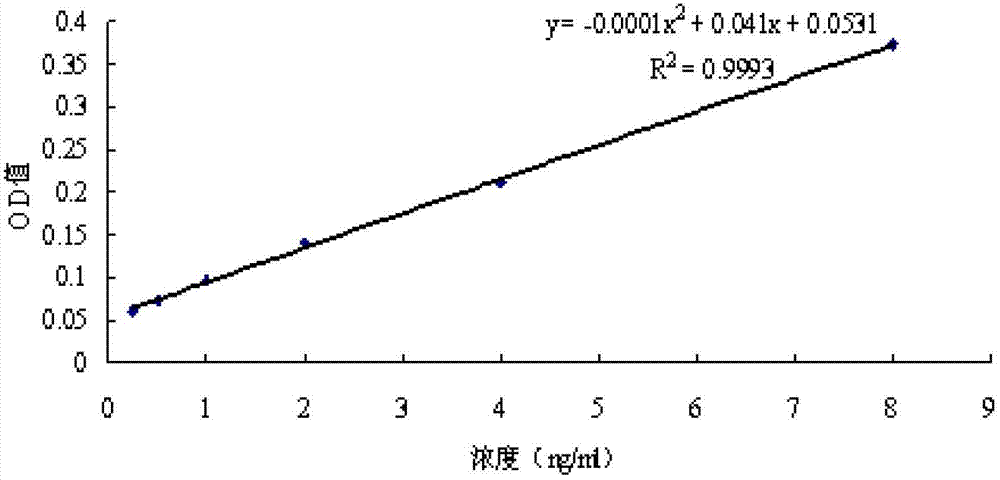
[0059] Example 3: Drugs inhibit the expression level of HBsAg in the culture supernatant of HepG2.2.15 cells
[0060] 1. Experimental materials
[0061] (1) Main reagents: HBsAg detection ELISA kit (Shanghai Yanji Biotechnology Co., Ltd.).
[0062] (2) Main instruments: automatic microplate reader (Bio-TEK, Elx800).
[0063] 2. Experimental method
[0064] The HepG2.2.15 cells (5×10 4 unit / mL) in CO 2 After culturing in the incubator for 24 hours, the cells adhered to the wall and grew well, and the culture medium was removed. According to the results of the cytotoxicity test, the cell culture medium containing different concentrations of drugs without G418 was added, and each concentration was set for 3 replicates. The cells in the complete medium were normal control, 5% CO 2 , Culture at 37°C. After 48h, 72h, and 96h of continuous culture, the supernatant of each sample was drawn and placed in a 1.5mL sterilized centrifuge tube, centrifuged at 1000rcf for 10min to take...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com