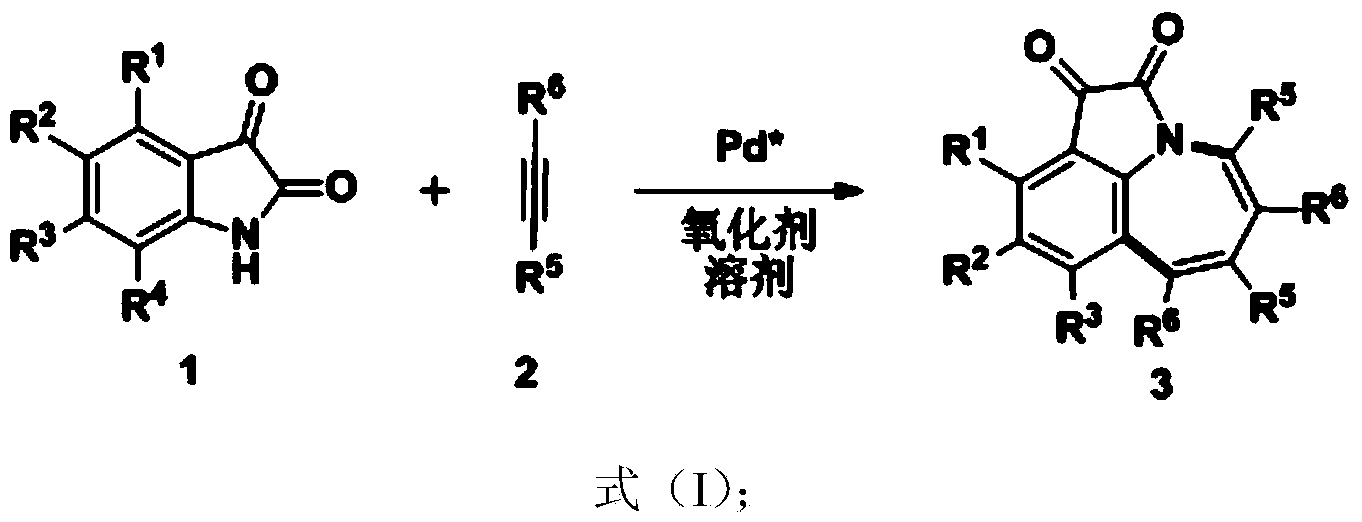
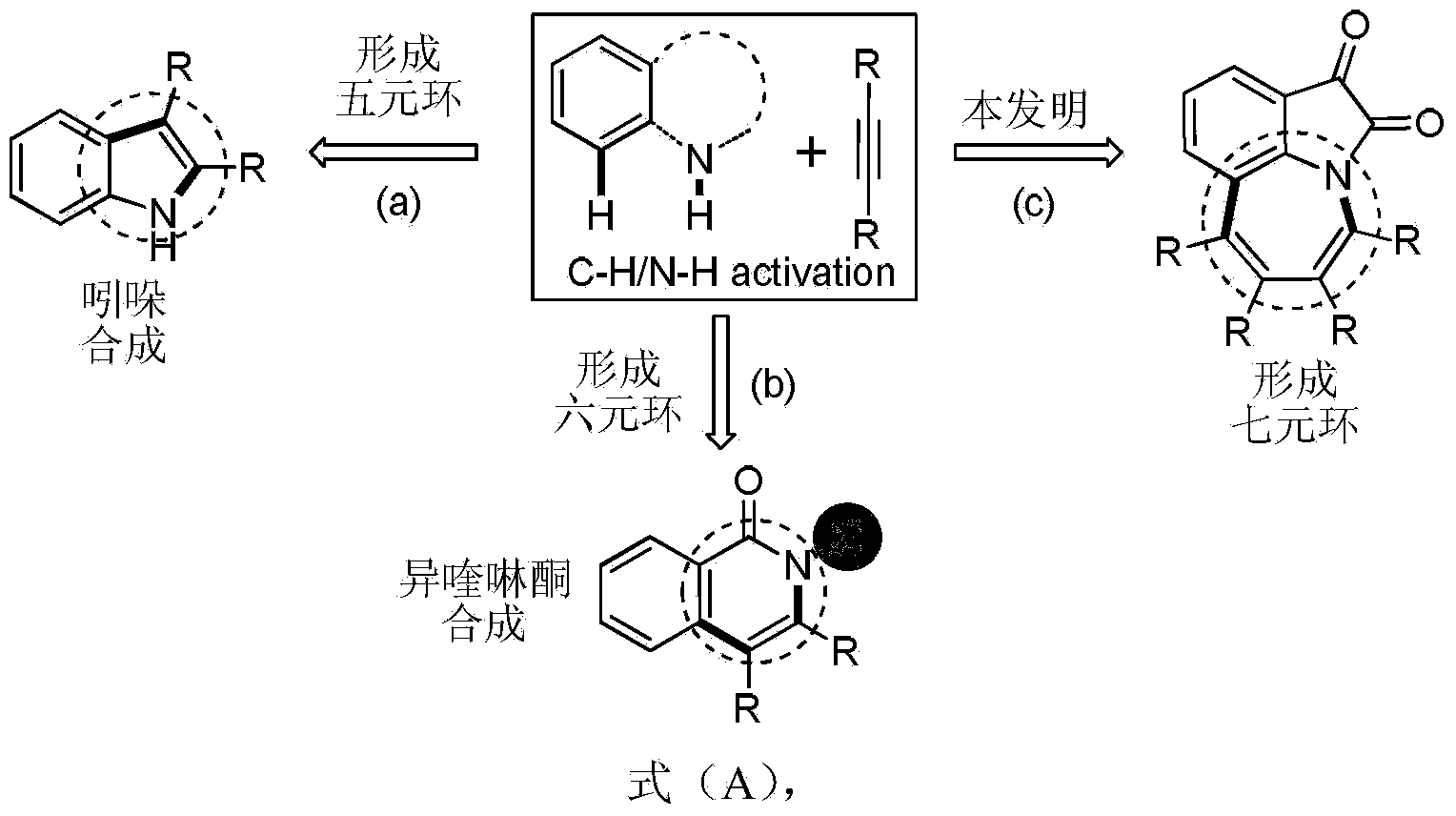
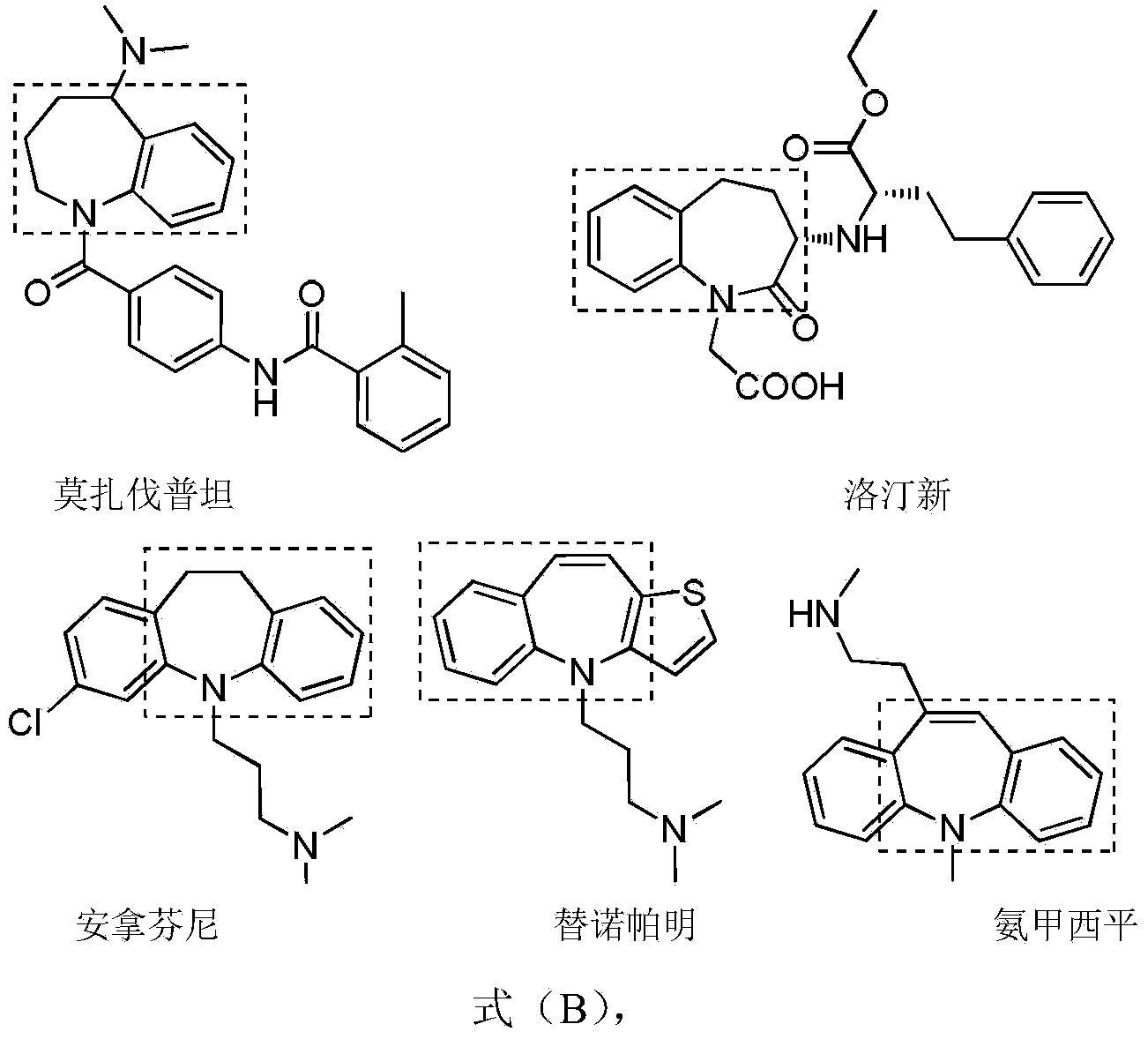
Method for synthesis of 1,2,3,4,5,9-substituted benzazepine compound
A technology of benzazepine and synthesis method, which is applied in the field of organic compound process application, can solve problems such as no synthesis method proposed, and achieve the effects of promoting deep-level expansion, stable properties, and less stringent storage conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023]
[0024] Under the protection of inert gas, isatin compound 1a (0.2mmol) and alkyne compound 2a (1.0mmol) were dissolved in acetonitrile / 1,4-dioxane (2mL) (v / v=1:1), Add palladium acetate (0.02mmol) and silver acetate (0.4mmol), and stir the reaction system at 100°C for 12 hours, and detect it by TLC. After the reaction, cool down to room temperature, extract several times with dichloromethane, combine the organic phases, dry, and rotate The crude product was obtained by removing the solvent, and the product 3aa was obtained by flash column chromatography with a yield of 81%. 1 H NMR (300MHz, CDCl 3 ): δ=7.56(dd, J=7.3, 1.2Hz, 1H), 7.23-6.92(m, 18H), 6.79-6.75(m, 4H); 13 C NMR (75MHz, CDCl 3 ):δ=181.83,161.85,160.11,145.38,141.57,140.51,140.24,139.62,138.22,137.25,135.87,131.39,130.79,130.76,130.67,130.42,128.39,128.27,128.19,127.58,127.48,127.07,126.86, 126.27, 124.68, 121.71; HRMS (ESI) calculated value C 36 h 24 NO 2 [M+H] + 502.1802, actual value 502.1801....
Embodiment 2
[0026]
[0027] The operating steps are the same as in Example 1, and the yield is 96%. 1 H NMR (500MHz, CDCl 3 ): δ=7.37(s, 1H), 7.13-6.87(m, 17H), 6.72(m, 4H), 2.24(s, 3H); 13 C NMR (125MHz, CDCl3 ):δ=182.03,162.12,158.11,145.26,142.09,140.59,140.49,140.37,139.55,138.33,136.91,136.23,135.88,131.42,130.77, 130.70,130.53,130.41,128.34,128.24,128.14,127.55,127.44, 127.41, 127.01, 126.79, 124.93, 121.79, 21.30; HRMS (ESI) calculated value C 37 h 26 NO 2 [M+H] + 516.1958, actual value 516.1965.
Embodiment 3
[0029]
[0030] The operating steps are the same as in Example 1, and the yield is 93%. 1H NMR (500MHz, CDCl3): δ=7.42(t, J=9.9Hz, 1H), 7.13-6.90(m, 17H), 6.79-6.72(m, 4H), 2.76(hept, J=6.9Hz, 1H ), 1.14 (d, J=6.9Hz, 6H); 13C NMR (125MHz, CDCl3): δ=182.14, 162.05, 158.30, 147.15, 145.00, 140.57, 140.42, 140.39, 139.71, 138.36, 136.90, 135.90, 131. 130.79,130.75,130.63,130.43,128.25,128.21,128.11,127.53,127.43,127.40,127.00,126.79,122.29,121.69,33.84,23.91;HRMS(ESI)计算值C39H30NO2[M+H]+544.2271,实际值544.2286 ..
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com