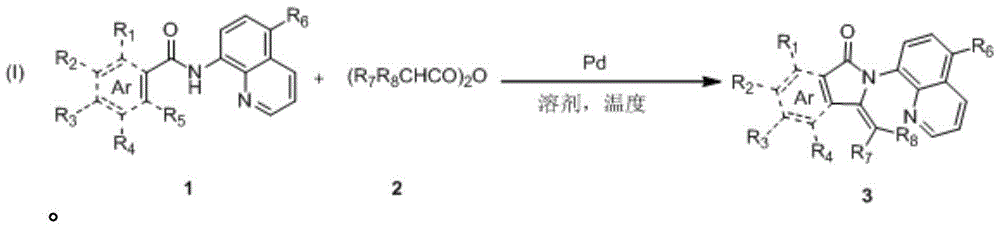
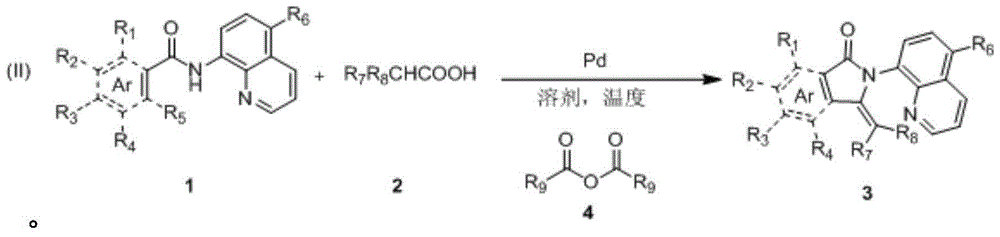
The synthetic method of 3-methylene isoindolone compound
A methylene isoindolinone and synthesis method technology, applied in the direction of organic chemistry, can solve the problems of reducing product structure diversity, cumbersome and lengthy operation steps, and poor functional group compatibility, so as to promote deep-level expansion and simple and easy raw materials Obtained and stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
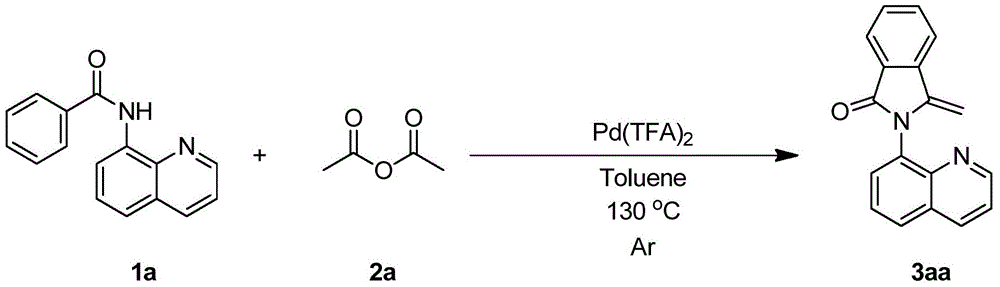
Embodiment 1
[0028]
[0029] Take a 20mL reaction tube, add the weighed benzamide compound 1a (0.1mmol), acetic anhydride 2a (0.2mmol), palladium trifluoroacetate (0.005mmol), and then add After toluene (1 mL), the reaction tube was sealed with a Teflon stopper. Place the reaction tube in an oil bath at 130°C and stir for 12 hours. After the reaction is complete, cool down to room temperature, filter with diatomaceous earth, and then rinse with 20 mL of ethyl acetate until the filtrate is colorless. Combine the organic phases and spin to remove the solvent to obtain The mixture containing 3-methylene-2-(quinolin-8-yl)isoindolin-1-one(3aa) was subjected to flash column chromatography to obtain the product 3aa in a yield of 99%.
[0030] 1 H NMR (600MHz, CDCl 3 )δ9.00–8.81(m,1H),8.24(d,J=8.3Hz,1H),7.97(t,J=8.6Hz,2H),7.79(dd,J=17.3,7.4Hz,2H), 7.71–7.63(m, 2H), 7.58(t, J=7.5Hz, 1H), 7.44(dd, J=8.3, 4.1Hz, 1H), 5.19(d, J=1.8Hz, 1H), 4.41(d ,J=1.8Hz,1H). 13 C NMR (150MHz, CDCl 3 )δ167.4...
Embodiment 2
[0032]
[0033] Operation steps are with embodiment 1, obtain product
[0034] 3-methylene-2-(quinolin-8-yl)-5-(trifluoromethyl)isoindolin-1-one(3ba), the yield is 80%.
[0035] 1 H NMR (600MHz, CDCl 3)δ8.89(dd, J=4.1,1.5Hz,1H),8.26(dd,J=8.3,1.3Hz,1H),8.10(d,J=7.9Hz,1H),8.06(s,1H), 7.99(dd, J=8.2,0.8Hz,1H),7.85(d,J=7.9Hz,1H),7.82–7.76(m,1H),7.70(t,J=7.7Hz,1H),7.46(dd ,J=8.3,4.1Hz,1H),5.29(d,J=2.4Hz,1H),4.53(d,J=2.4Hz,1H). 13 C NMR (150MHz, CDCl 3 )δ166.1, 151.2, 144.5, 143.0, 136.9, 136.3, 134.2 (q, 2 J C-F =32.4Hz), 132.2, 132.0, 130.8, 129.6, 129.5, 126.5 (q, 3 J C-F =3.6Hz), 126.2, 124.7(q, 1 J C-F =271.2Hz), 124.3, 121.9, 117.7(q, 3 J C-F =3.9Hz), 92.0; (ESI) calculated value C 19 h 11 f 3 N 2 O[M+Na] + 363.0721, actual value 363.0721.
Embodiment 3
[0037]
[0038] Operation steps are with embodiment 1, obtain product
[0039] 5-methyl-3-methylene-2-(quinolin-8-yl)isoindolin-1-one(3ca), yield 99%.
[0040] 1 H NMR (600MHz, CDCl 3 )δ8.89(dd, J=4.0,1.5Hz,1H),8.23(dd,J=8.3,1.4Hz,1H),7.95(dd,J=8.2,0.9Hz,1H),7.86(d,J =7.7Hz,1H),7.76(dd,J=7.2,1.0Hz,1H),7.67(t,J=7.7Hz,1H),7.59(s,1H),7.43(dd,J=8.3,4.1Hz ,1H),7.39(d,J=7.7Hz,1H),5.14(d,J=2.0Hz,1H),4.36(d,J=1.9Hz,1H),2.53(s,3H). 13 C NMR (150MHz, CDCl 3 C 19 h 14 N 2 O[M+H] + 287.1184, actual value 287.1185.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com