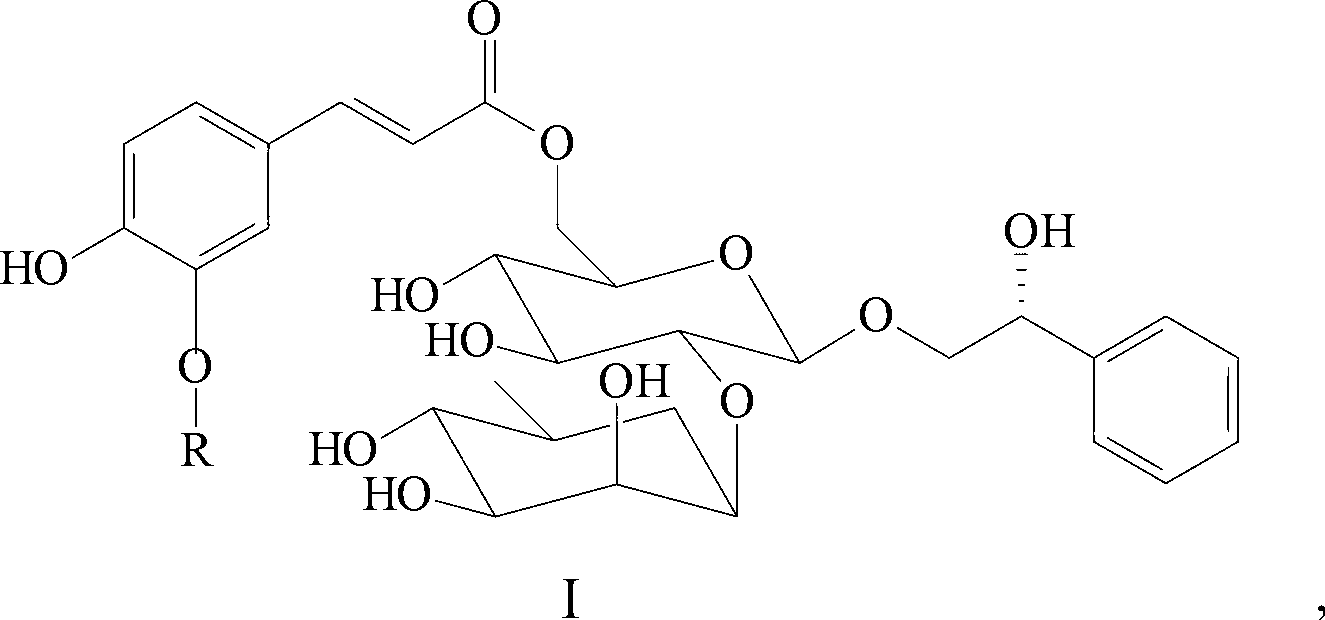
Application of phenylethanoid glycoside monomer compounds
A compound and drug technology, applied in the field of medicine, can solve problems such as unclear effects of phenylethanol glycoside monomers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation of embodiment 1, savatiside E
[0030] (1) Extraction of medicinal materials
[0031] 50kg of velvet antler medicinal material, cut into small pieces of 1-2cm, heated and refluxed with 15 times the amount of water to extract three times, each time for 2 hours. Combine the extracts, concentrate under reduced pressure at 70°C to 50L (relative density 1.15-1.18), add 250L of 95% ethanol, stir while adding, let stand overnight, take the supernatant, and concentrate under reduced pressure at 70°C to 50L.
[0032] (2) AB-8 resin purification
[0033] Resin dosage: medicinal material quality / resin quality=1:1;
[0034] Sample loading speed: 1mL / min.cm 2 ;
[0035] Elution rate: 2-3mL / min.cm 2 ;
[0036] The diameter-to-height ratio of the resin bed is 1:10;
[0037] Take 50 kg of the processed AB-8 resin, put it into a stainless steel chromatographic column with a diameter of 30 cm, wash it with water, and set it aside. The extract was loaded twice and ...
Embodiment 2
[0058] The preparation of embodiment 2, savatiside A
[0059] (1) Extraction of medicinal materials
[0060] 50kg of velvet antler medicinal material, cut into small pieces of 1-2cm, heated and refluxed with 15 times the amount of water to extract three times, each time for 2 hours. Combine the extracts, concentrate under reduced pressure at 70°C to 50L (relative density 1.15-1.18), add 250L of 95% ethanol, stir while adding, let stand overnight, take the supernatant, and concentrate under reduced pressure at 70°C to 50L.
[0061] (2) AB-8 resin purification
[0062] Resin dosage: medicinal material quality / resin quality=1:1;
[0063] Sample loading speed: 1mL / min.cm 2 ;
[0064] Elution rate: 2-3mL / min.cm 2 ;
[0065] The diameter-to-height ratio of the resin bed is 1:10;
[0066] Take 50 kg of the processed AB-8 resin, put it into a stainless steel chromatographic column with a diameter of 30 cm, wash it with water, and set it aside. The extract was loaded twice and ...
Embodiment 3
[0087] Embodiment 3, DPPH method measures the scavenging ability of phenylethanoid glycoside monomer compound to free radical
[0088] The savatiside E prepared in Example 1 and the savatiside A prepared in Example 2 were added with distilled water to form a 1 mg / mL sample solution, and then diluted to 100 μg / mL, 50 μg / mL, 25 μg / mL, 12.5 μg / mL, 6.25μg / mL and 3.125μg / mL and six concentrations are available for use. At the same time, 100 μg / mL vitamin E (VE) solution was used as a positive control.
[0089] Take 100 μL of the above-mentioned sample solutions of different concentrations in a 96-well ELISA plate, make three parallel wells for each concentration, then add 100 μL of 1 mM DPPH test solution, shake for 30 seconds, and measure the absorbance of each well at 37°C and 517nm wavelength (Ap). Simultaneously measure the blank absorbance value (Ac) of the sample solution without DPPH and the absorbance value (A max) of the sample solution with DPPH but no sample solution (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com