Preparation and application of amino substituted carboxylic acid compounds
A technology of compounds and carboxylic acids, which is applied in the field of two new types of amino-substituted carboxylic acid compounds and their preparation, can solve the problems of unsatisfactory effect of promoting drainage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
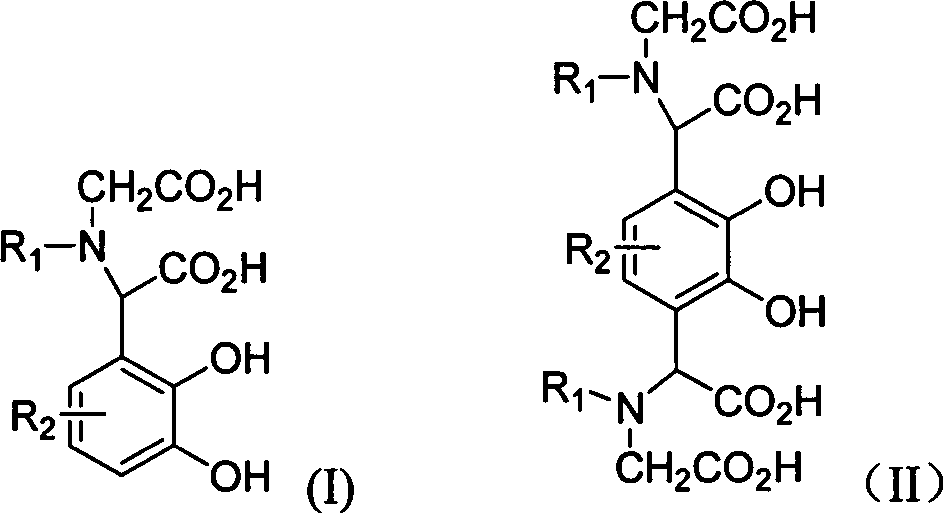
[0020] Example 1 compound 2-carbonylmethylamino-2-2', 3'-dihydroxyphenylacetic acid (4, R in general formula (I) 1 = H, R 2 =H) Preparation
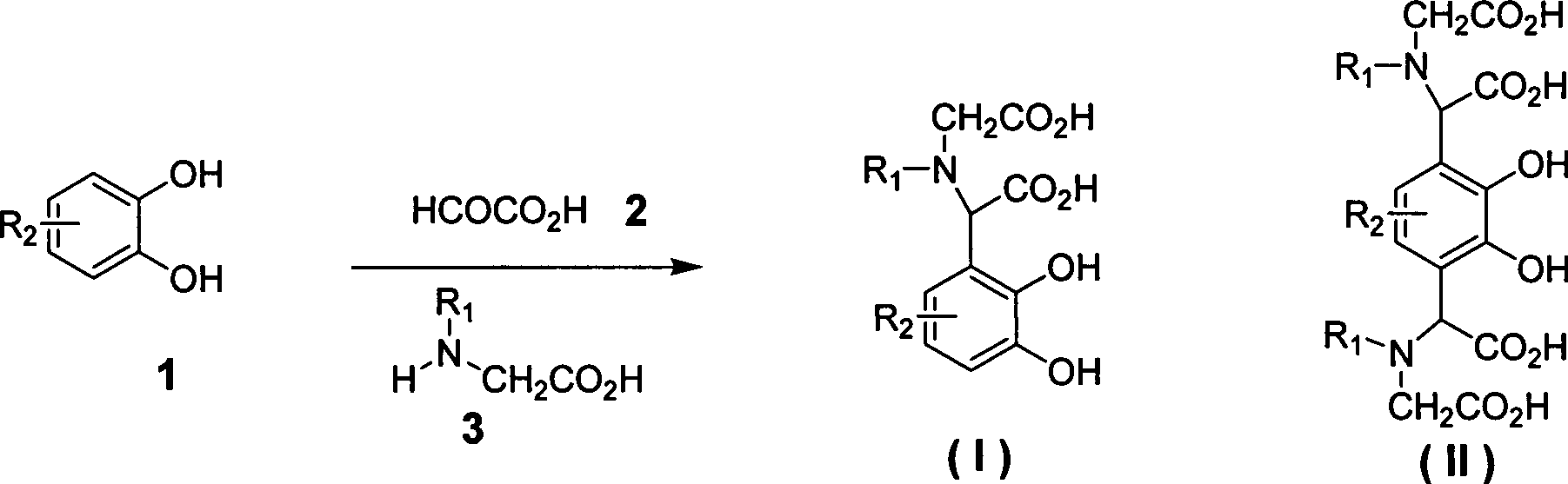
[0021] Add catechol (1, R 2 =H) 2.2g, 2-oxoacetic acid (2) 1.84g, 2-aminoacetic acid (3, R 1 =H) 1.65g and water 20mL, mix well, add 0.5mL acetic acid dropwise, heat to reflux for reaction (TLC detection end point), cool, filter with suction, wash with a small amount of ethanol, and dry to obtain 2.26g of white solid. Y=47%; mp: 220°C (decomposition); 1 H NMR (DMSO-d 6 , 400MHz): δ7.41 (d, J=8.2Hz, 1H), 6.80-7.21 (m, 2H), 3.23-3.11 (m, 3H) ppm; MS (m / z, ESI - ): 240[M-H] - ;IR (KBr): 3430, 3021, 2980, 1687, 1679, 1219cm -1 .
Embodiment 2
[0022] Example 2 Compound 2-N, N-dicarbonylmethylamino-2-2', 3'-dihydroxy-5'-methoxyphenylacetic acid (5, R in the general formula (I) 1 =CH 2 CO 2 H, R 2 =OCH 3 ) preparation
[0023] Add substituted catechol (1, R 2 =4-methoxy) 11.2g, 2-oxoacetic acid (2) 7.36g, iminodiacetic acid (3, R 1 =CH 2 CO 2 H) 10.64g and 80mL of water, mix well, add 0.5mL of acetic acid dropwise, heat to reflux for reaction (TLC detection end point), cool and suction filter to obtain 6.9g of white solid. Y=53%; mp: 250°C (decomposition); 1 H NMR (DMSO-d 6 , 400MHz): δ7.35-7.24 (m, 2H), 3.87 (s, 3H), 3.43 (s, 1H), 3.32-3.21 (m, 4H) ppm; MS (m / z, ESI - ): 328[M-H] - ;IR (KBr): 3430, 3052, 2992, 2635, 1695, 1637, 1210cm -1 .
Embodiment 3
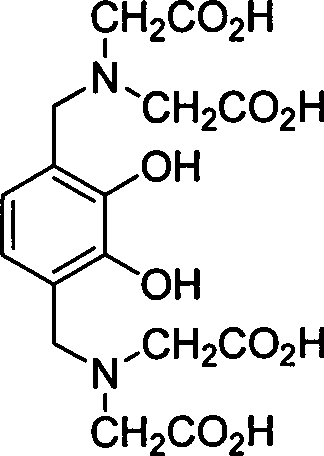
[0024] Example 3 compound 2,2'-N,N-dicarbonylmethylamino-2',3'-dihydroxy 1',4'-phenyl diacetic acid (6, R in general formula (II) 1 =CH 2 CO 2 H, R 2 =H) Preparation
[0025] Add catechol (1, R 2 =H) 4.4g, 2-oxoacetic acid (2) 7.36g, iminodiacetic acid (3, R 1 =CH 2 CO 2 H) 10.64g and 70mL of water, after mixing evenly, add 0.5mL of acetic acid dropwise, heat to reflux for reaction (TLC detection end point), cool and filter with suction to obtain 6.8g of white solid. Y=70%; mp: 250°C (decomposition); 1 H NMR (DMSO-d 6 , 400MHz): δ7.42 (d, J=8.6Hz, 2H), 3.41 (s, 2H), 3.40-3.23 (m, 8H) ppm; MS (m / z, ESI - ): 487[M-H] - ;IR (KBr): 3435, 3052, 2987, 2685, 1701, 1629, 1265cm -1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com