Method for synthesizing isopropyl-beta-D-thiogalactoside
A technology of thiogalactoside and thiogalactoside, which is applied in the field of synthesis of isopropyl-β-D-thiogalactoside, can solve the problems of fire-prone handling workload and environmental burden, and achieve process safety The effect of convenience and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
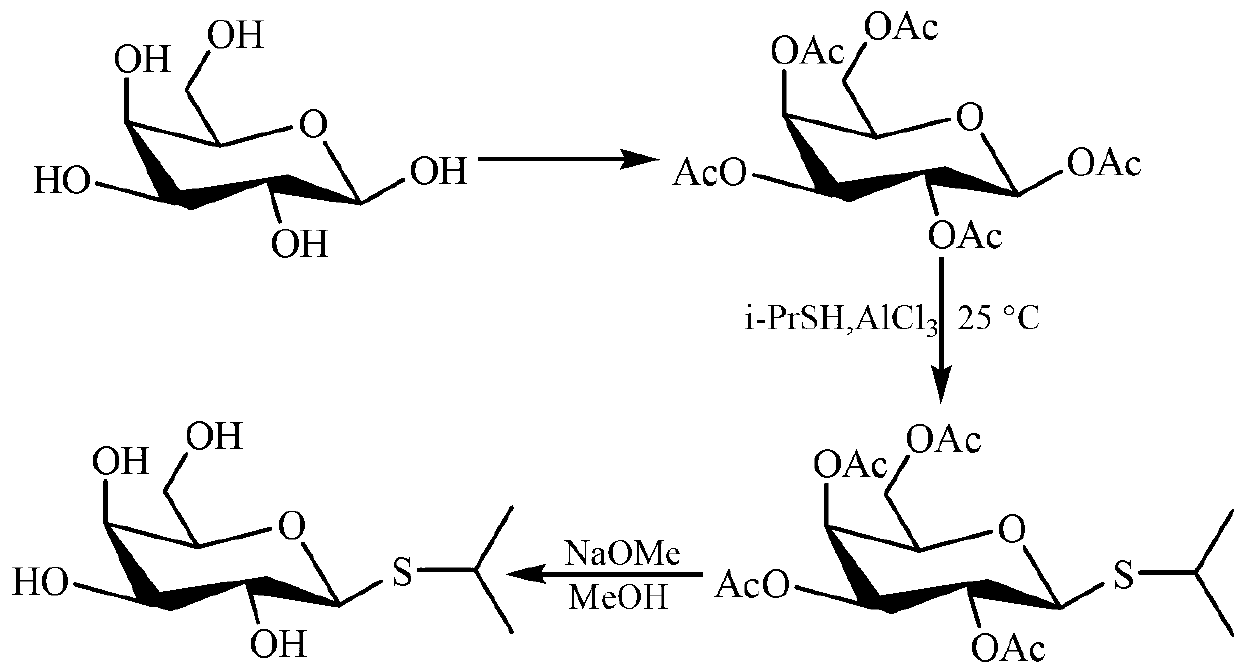
[0014] Example 1 Preparation of isopropyl-β-D-thiogalactoside
[0015] Preparation of penta-O-acetyl-β-D-galactose
[0016] 1. Add 125 ml of acetic anhydride and 20 g of anhydrous sodium acetate into a 500 ml reaction flask, slowly heat to reflux to dissolve the solid completely, and then carefully add 25 g of D-galactose in batches. After the addition was complete, the reaction mixture was refluxed for an additional 120 minutes. Afterwards, the reaction solution was poured into 300 ml ice-water mixture and stirred vigorously for 30 minutes, and a khaki solid precipitated out. Filter, wash the filter cake with water 2-3 times, and dry below 70°C. The obtained 37.9 g of penta-O-acetyl-β-D-galactose was recrystallized with ethanol as a solvent, and dried to obtain 31.1 g of pure penta-O-acetyl-β-D-galactose as a colorless solid , the yield is about 57%.
[0017] 2. Add 150 ml of acetic anhydride and 20 g of anhydrous sodium acetate into a 500 ml reaction flask, slowly heat t...
Embodiment 2
[0023] Example 2 Preparation of isopropyl-β-D-thiogalactoside
[0024] Preparation of penta-O-acetyl-β-D-galactose
[0025]1. Add 125 ml of acetic anhydride and 20 g of anhydrous sodium acetate into a 500 ml reaction flask, slowly heat to reflux to dissolve the solid completely, and then carefully add 25 g of D-galactose in batches. After the addition was complete, the reaction mixture was refluxed for an additional 140 minutes. Afterwards, the reaction solution was poured into 300 ml ice-water mixture and stirred vigorously for 35 minutes, and a khaki solid precipitated out. Filter, wash the filter cake with water 2-3 times, and dry below 70°C. The obtained 37.9 g of penta-O-acetyl-β-D-galactose was recrystallized with ethanol as a solvent, and dried to obtain 31.1 g of pure penta-O-acetyl-β-D-galactose as a colorless solid , the yield is about 58%.
[0026] 2. Add 150 ml of acetic anhydride and 20 g of anhydrous sodium acetate into a 500 ml reaction flask, slowly heat to...
Embodiment 3
[0032] Example 3 Preparation of isopropyl-β-D-thiogalactoside
[0033] Preparation of penta-O-acetyl-β-D-galactose
[0034] 1. Add 125 ml of acetic anhydride and 20 g of anhydrous sodium acetate into a 500 ml reaction flask, slowly heat to reflux to dissolve the solid completely, and then carefully add 25 g of D-galactose in batches. After the addition was complete, the reaction mixture was refluxed for an additional 120 minutes. Afterwards, the reaction solution was poured into 300 ml ice-water mixture and stirred vigorously for 30 minutes, and a khaki solid precipitated out. Filter, wash the filter cake with water 2-3 times, and dry below 70°C. The obtained 37.9 g of penta-O-acetyl-β-D-galactose was recrystallized with ethanol as a solvent, and dried to obtain 31.1 g of pure penta-O-acetyl-β-D-galactose as a colorless solid , the yield is about 57%.
[0035] 2. Add 150 ml of acetic anhydride and 20 g of anhydrous sodium acetate into a 500 ml reaction flask, slowly heat t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com