Piglet colibacillosis lyophilized serum immunoglobulin preparation and preparation process thereof
A technology of colibacillosis and serum immunity, which is applied in the direction of serum immunoglobulin, antibacterial immunoglobulin, freeze-dried delivery, etc., can solve the problems of difficult clinical wide acceptance, high price, and difficult implementation of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
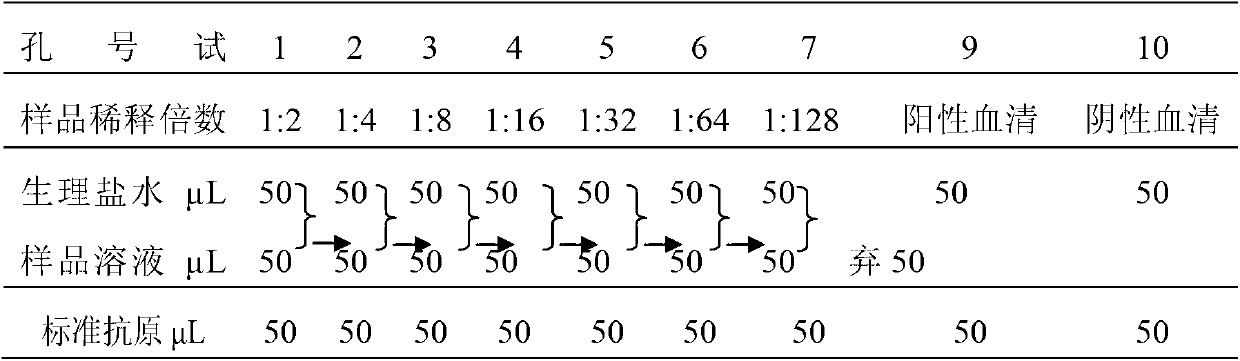
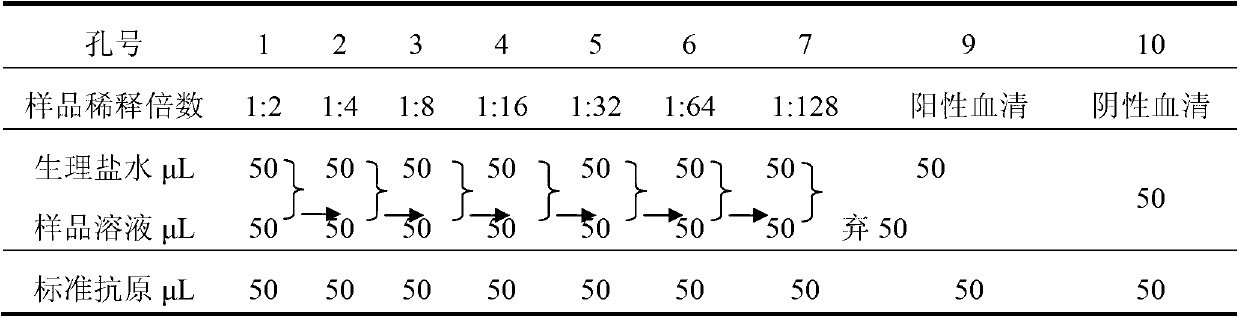
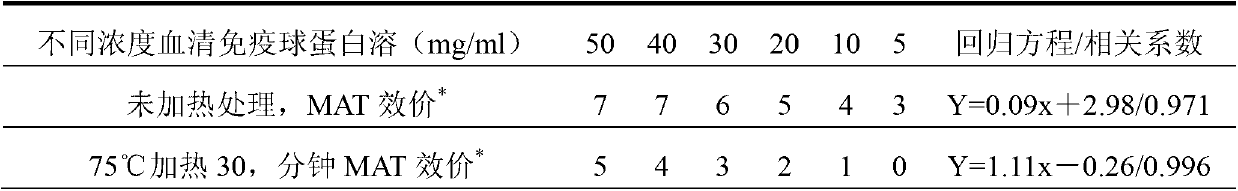
[0179] The infectious agent of this preparation is Escherichia coli (ETEC K88 K99 987P).
[0180] The components and parts by weight of the piglet colibacillosis freeze-dried serum immunoglobulin preparation of the present embodiment are as follows:
[0181] Saccharoterpenoids 12.0
[0182] Scopolamine 0.008
[0183] Serum immunoglobulin 88.0
[0184] The preparation of this example was prepared by the following method:
[0185] 1. Preparation of saccharoterpenoids
[0186] (1) Sugar extraction:
[0187] a. Take Atractylodes Rhizoma Atractylodes 40, Atractylodes Rhizoma 30, Muxiang 20 and Alisma orientalis 10 in proportion by weight, chopped, washed, soaked in cold water for 2 hours until the core of the medicine is fully soaked, then add water 12 times the total weight of the traditional Chinese medicine, and heat to After boiling, under the condition of 90 ℃, decoct for 180 minutes under the heat reflux concentration process and concentrate to obtain concentrated medici...
Embodiment 2
[0225] The infectious agent of this preparation is Escherichia coli (ETEC K88 K99 987P).
[0226] The components and parts by weight of the piglet colibacillosis freeze-dried serum immunoglobulin preparation of the present embodiment are as follows:
[0227] Saccharoterpenoids 55
[0228] Scopolamine 0.005
[0229] Serum immunoglobulin 55.0
[0230] The preparation method is basically the same as that of Example 1, except that the sugars and triterpenoid saponins in the saccharoterpenes are extracted from the following traditional Chinese medicines in parts by weight: Atractylodes Rhizoma 30, Atractylodes Rhizoma 40, Muxiang 15 and Alisma 15.
[0231] The quality standard is the same as that of Example 1.
Embodiment 3
[0233] The infectious agent of this preparation is Escherichia coli (ETEC K88 K99 987P).
[0234] The components and parts by weight of the piglet colibacillosis freeze-dried serum immunoglobulin preparation of the present embodiment are as follows:
[0235] Saccharoterpenoids 30
[0236] Scopolamine 0.0055
[0237] Serum Immunoglobulin 70
[0238] The preparation method is basically the same as in Example 1, except that the sugars and triterpenoid saponins in the saccharoterpenoids are all extracted from the following traditional Chinese medicines in parts by weight: Atractylodes Rhizoma 50, Atractylodes Rhizoma 20, Muxiang 15 and Alisma 15.
[0239] The quality standard is the same as that of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com