Preparation method and device for direct phase change heat transfer type gas hydrates
A phase change heat and hydration technology, applied in mixing methods, chemical methods for reacting liquid and gaseous media, materials for heat exchange, etc. Operation, easy constant temperature operation, temperature rise suppression effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
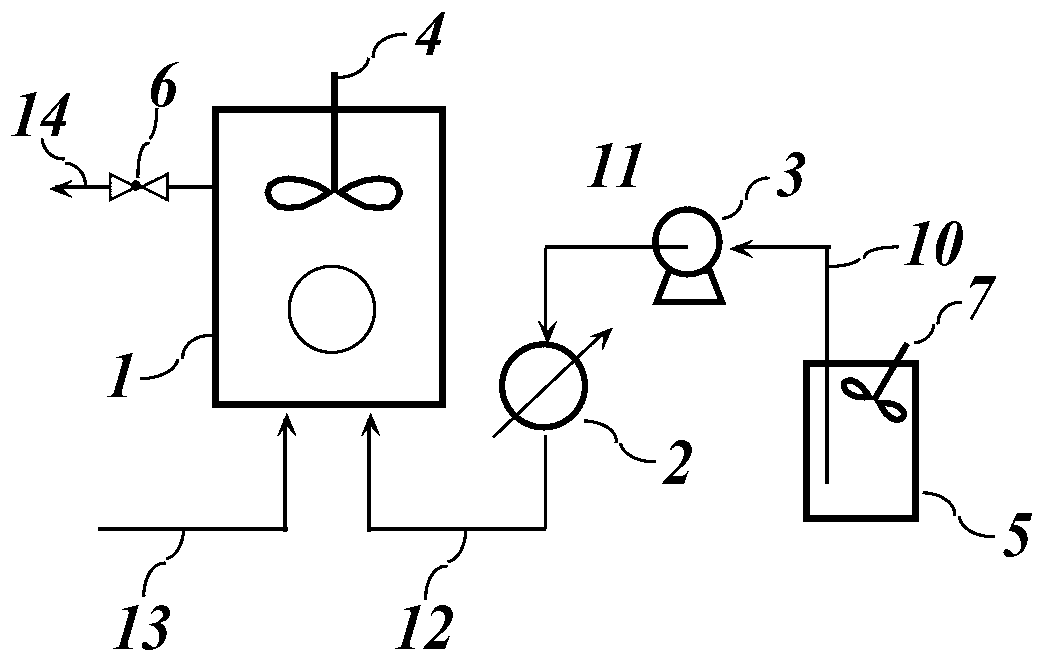
[0036] Used devices such as figure 1 Shown: the disperser 7 is used for high-speed shearing of a mixed liquid containing phase change materials, surfactants, nucleating agents and water. Fix the disperser 7 on the top of the container 5 with a bracket, adjust the height of the disperser 7 so that the cutter head of the disperser 7 extends into the liquid in the container 5, and the liquid in the container 5 passes under the action of the pressure pump 3. The suction line 10 and the discharge line 11 are transported to the heat exchanger 2. The heat exchanger 2 is connected to the hydrator 1 through the inlet line 12, and the bottom of the hydrator 1 is provided with an air inlet, and the hydrated gas passes through the air inlet line 13 Enter the hydrator 1, which is equipped with a stirrer 4, which is mainly used to strengthen the mass transfer between gas and liquid and enhance the phase change heat transfer in the hydration process, and try to avoid the formation of hydrate ...
Embodiment 2
[0040] Select the phase change material normal carbon tetradecane, deionized water, surfactant span60 and tween60 and the nucleating agent normal octadecane to make the phase change material 35wt%, water 62.2wt%, surfactant 2wt%, The nucleating agent is a 0.8wt% mixed liquid, in which the mass ratio of span60 and tween60 is 1:2, and this liquid mixing process is completed in container 5. Then the container 5 containing the mixed solution was placed in a constant temperature water bath at 30°C for half an hour. After the temperature stabilized, the disperser 7 was used for processing at 10000 rpm for one hour to obtain an emulsion of the phase change material and water. The emulsion is sent to the heat exchanger 2 by the pressure pump 3 through the suction line 10 and the discharge line 11 at a flow rate of 4 mL / min. After cooling, the phase change material droplets in the emulsion solidify to form a solid particle dispersed in water. Slurry. The slurry enters the hydrator 1 th...
Embodiment 3
[0042] Select the phase change material normal carbon tetradecane and normal carbon pentadecane, deionized water, surfactant span60 and tween 60 and nucleating agent normal octadecane to make the phase change material 60wt%, water 35wt%, surface A mixture of 4wt% active agent and 1wt% nucleating agent, in which the mass ratio of n-carbon tetradecane and n-carbon pentadecane is 4:1, and the mass ratio of span60 and tween60 is 1:2. This liquid mixing process Finished in container 5. Then the container 5 containing the mixed solution was placed in a constant temperature water bath at 30°C for half an hour, and after the temperature stabilized, the disperser 7 was used for processing at a speed of 30,000 rpm for 20 minutes to obtain an emulsion of phase change material and water. The emulsion is sent to the heat exchanger 2 by the pressure pump 3 through the suction line 10 and the discharge line 11 at a flow rate of 2.5 mL / min. After cooling, the phase change material droplets in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com