2-Acetylpyridine adenoaminophenol molybdenum complex and preparation method thereof
A technology of acetylpyridine and o-aminophenol, which is applied in the field of 2-acetylpyridine condensed o-aminophenol molybdenum complex and its preparation field, can solve problems such as no molybdenum Schiff base complex, and achieve excellent catalytic performance, Good stability and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Weigh 10 millimoles of o-aminophenol, put it into a round-bottomed flask (50 milliliters) containing 20 milliliters of absolute ethanol, heat and stir at 50° C., add 10 millimoles of 2-acetylpyridine after the o-aminophenol is completely dissolved, and stir for 1 After standing for one hour, light yellow polyhedral crystals were precipitated after 6 days. Filtrate with suction and wash with a small amount of ethanol to obtain 2-acetylpyridine o-aminophenol Schiff base.
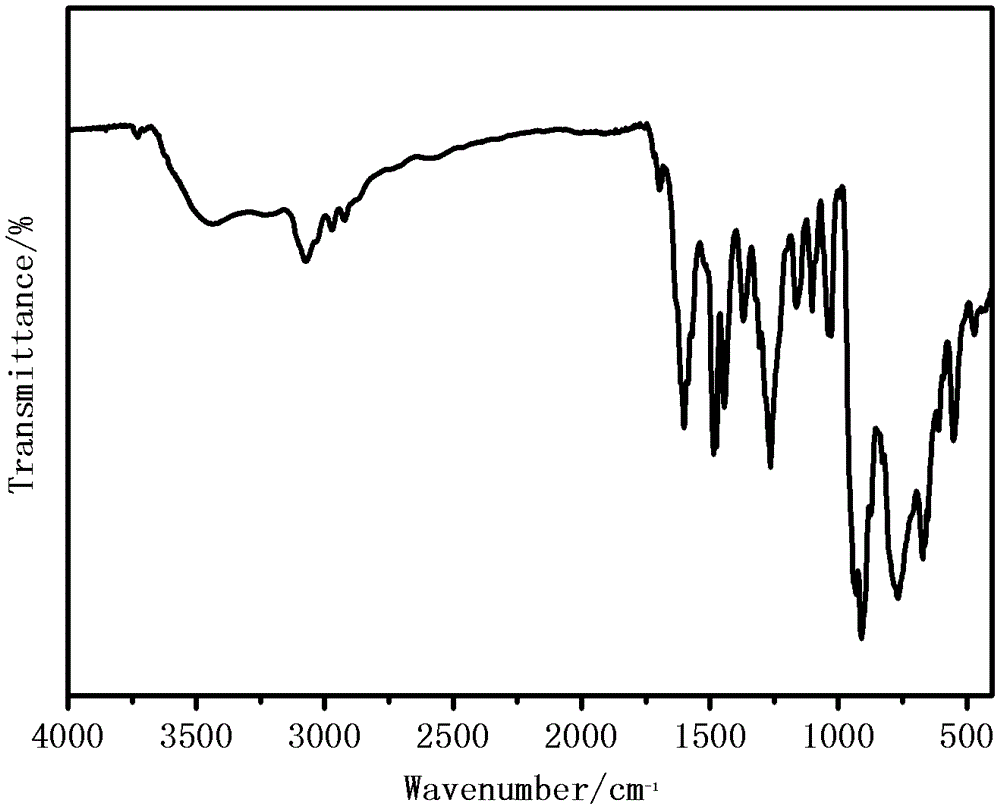
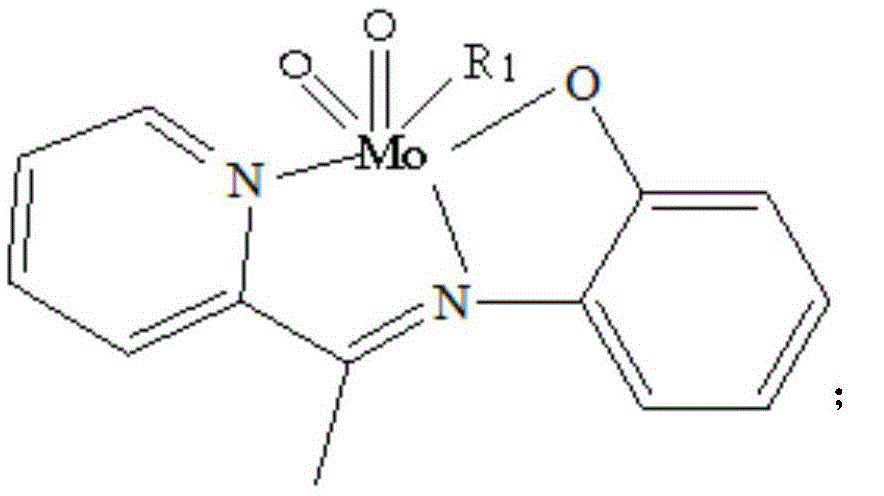
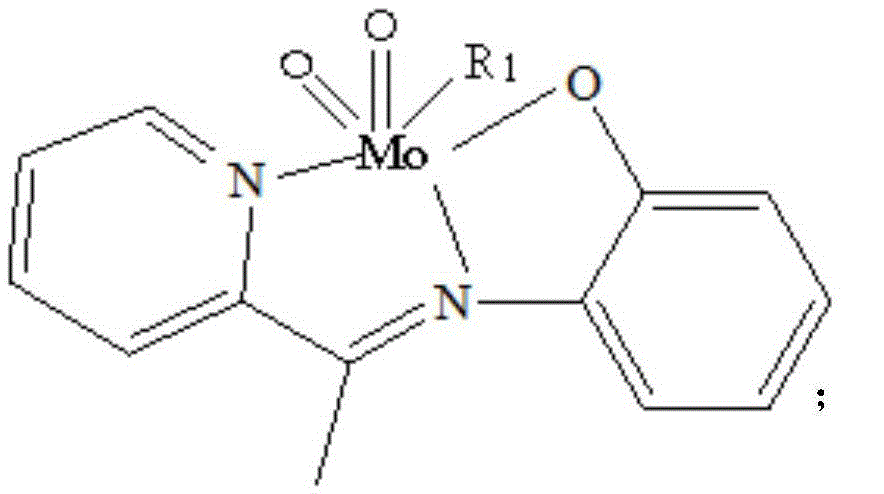
[0019] Weigh 5mmol of 2-acetylpyridine-o-aminophenol, put it into a round-bottomed flask (50ml) containing 15ml of absolute ethanol, heat and stir at 75°C, and add 5mmol of acetylacetone after the 2-acetylpyridine-o-aminophenol is completely dissolved Molybdenum, stirred brown powder to precipitate, continue to heat and stir for 2 hours, then cool and suction filter, wash with ethanol and dry in vacuum at 40°C to obtain a brown powder product, its structural formula is as follows, and its infrared analy...
Embodiment 2
[0022] Weigh 10 mmol of o-aminophenol, put it into a round-bottomed flask (50 ml) containing 20 ml of pyridine, heat and stir at 50°C, add 10 mmol of 2-acetylpyridine after the o-aminophenol is completely dissolved, and stir for 1 hour After standing still, light yellow polyhedral crystals were precipitated after 6 days. Filtrate with suction and wash with a small amount of ethanol to obtain 2-acetylpyridine o-aminophenol Schiff base.
[0023] Weigh 5mmol of 2-acetylpyridine-o-aminophenol, put it into a round-bottomed flask (50ml) containing 15ml of pyridine, heat and stir at 75°C, and add 5mmol of molybdenum acetylacetonate after the 2-acetylpyridine-o-aminophenol is completely dissolved. Stir brown powder to precipitate, continue to heat and stir for 2 hours, then cool and suction filter, wash with pyridine and dry in vacuum at 40°C to obtain the product. Its structural formula is as follows, and its infrared analysis spectrum is consistent with figure 1 resemblance.
[00...
Embodiment 3
[0026] Take tert-butyl hydroperoxide, n-propanol and the 2-acetylpyridine adenoaminophenol molybdenum complex synthesized in [Example 1] and put them into a high-pressure reactor, seal the reactor and start heating and stirring. When the preset 100°C is reached, the high-pressure pump pumps in propylene, and the amount of propylene is determined by the pumped volume. Reaction timing, after reaching 2 hours, take off the reactor, cool to 10-15°C with ice water, and take the liquid phase into a frozen sampler. Continue to freeze the sampler at minus 20°C for 30 minutes, then unscrew the air valve, slowly release the propylene in the sampler, open the sampler, quickly transfer the remaining liquid to the chromatographic sampling bottle, seal it, and perform chromatographic analysis.
[0027] Reaction conditions: reaction temperature 100°C, reaction time 2 hours, rotating speed 540rpm, tert-butyl hydroperoxide (TBHP) addition 70 millimoles, n-propanol addition 50 millimoles, catal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com