Method for anhydrously preparing isoindoline pigment
A technology of isoindoline and pigments, which is applied in the production process field of isoindoline yellow pigments, can solve the problems of difficult recycling, many by-products, and low product yields, and achieve industrial production convenience, low environmental pollution, The effect of high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
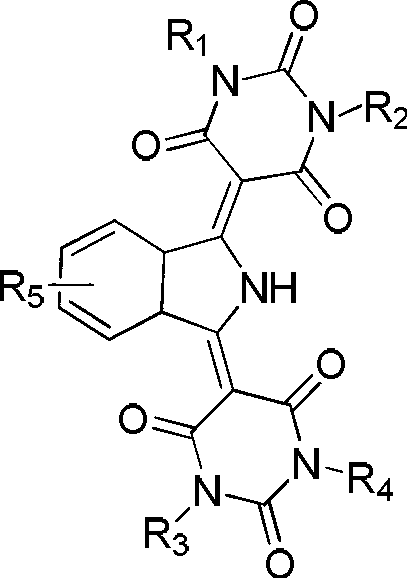
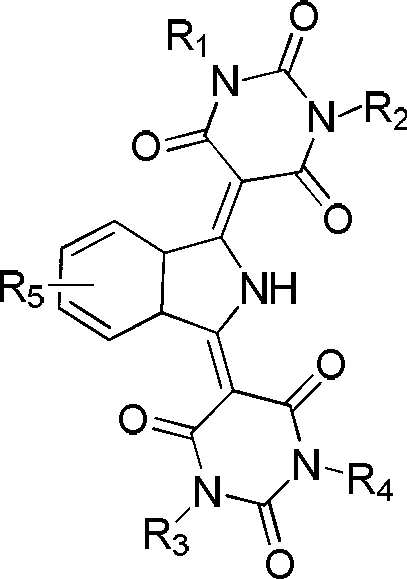
[0021] Add 800ml of N,N-dimethylformamide and 37.5g (0.293 moles) of barbituric acid into the reaction kettle, heat up to 55°C to dissolve, add 19.2g (0.132 moles) of 1,3-bis The iminoisoindoline was dissolved in a solution of 200ml N,N-dimethylformamide, and reacted for 30 hours, during which the ammonia gas produced was decompressed and fractionated. Cool down to 20-30°C, filter, wash with methanol, and dry in vacuum to obtain 46g of pigment with a yield of 95%.
Embodiment 2
[0023] Add 800ml of N,N-dimethylacetamide and 37.5g (0.293 moles) of barbituric acid into the reaction kettle, heat up to 140°C to dissolve, add 19.2g (0.132 moles) of 1,3-di The iminoisoindoline was dissolved in a solution of 200ml N,N-dimethylacetamide, and reacted for 15 hours. During the reaction, the ammonia gas produced was fractionally distilled off under reduced pressure. Cool down to 20-30°C, filter, wash with methanol, and dry in vacuum to obtain 45g of pigment with a yield of 94%.
Embodiment 3
[0025] Add 800ml of ethylene glycol and 37.5g (0.293 moles) of barbituric acid into the reaction kettle, heat up to 110°C to dissolve, add 19.2g (0.132 moles) of 1,3-diiminoisoindoline dropwise within 30 minutes Dissolved in 200ml of ethylene glycol, reacted for 25 hours, during the reaction process, the ammonia gas produced was decompressed and fractionated. Cool down to 20-30°C, filter, wash with methanol, and dry in vacuum to obtain 45g of pigment with a yield of 94%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com