Antagonist anti-il-7 receptor antibodies and methods
An IL-7R, antagonistic technology, applied in the direction of antibodies, anti-receptors/cell surface antigens/cell surface determinant immunoglobulins, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0298] Example 1: Production and screening of antagonistic IL-7R antibodies
[0299] This example illustrates the generation and screening of antagonist IL-7R antibodies.
[0300] General procedure for immunizing animals to produce monoclonal antibodies:
[0301] Two-month-old female Sprague Dawley rats were immunized with 50 μg of recombinant mouse IL-7Rα / CD127 / Fc chimera, which contained mouse IL-7Rα (Glu21-Asp239), hCD33 signal peptide (Met 1-Ala 16 ) and human IgG (Pro100-Lys330) (R&D Systems Cat. No. 747-MR). Antigen for immunization was prepared by mixing 50 μg antigen in 100 μl PBS with 100 μl Sigma Adjuvant System (Cat. No. S6322). The antigen mixture was swirled and injected into the hindlimb pads and peritoneum on days 0, 2, 5 and 7. On day 9, 50 μg of antigen in a total volume of 150 μl in physiological saline was injected intravenously without adjuvant. On day 13, splenocytes were prepared as single cell suspensions and fused with P3x63Ag8.653 mouse myeloma c...
Embodiment 2
[0319] Example 2: Determining Antibody Binding Affinity
[0320] This example illustrates determining the antibody binding affinity of antagonist IL-7R antibodies.
[0321] The affinity of an antagonistic IL-7R antibody for human IL-7R was measured on a surface plasmon resonance Biacore equipped with a research-grade CM5 sensor chip TM 2000 or 3000 biosensors (Biacore TM AB, Uppsala, Sweden–now GE Healthcare). Using standard N-hydroxysuccinimide / ethyldimethylaminopropylcarbodiimide (NHS / EDC) chemistry in HBS-P running buffer (from Biacore TM ), a goat polyclonal anti-human F(ab')2 fragment (Fc-specific) was ammonia-coupled to all four flow cells at saturating levels. The buffer was exchanged with HBS-P containing 1 mg / mL BSA. Human IL-7R-hFc antigen (R&D systems, Minneapolis, USA) was diluted to approximately 30 μg / mL and captured at 5 μL / min for 3 minutes to give a level of approximately 500-1000 RU per flow cell, leaving a blank as a reference aisle. Antibodies in ...
Embodiment 4
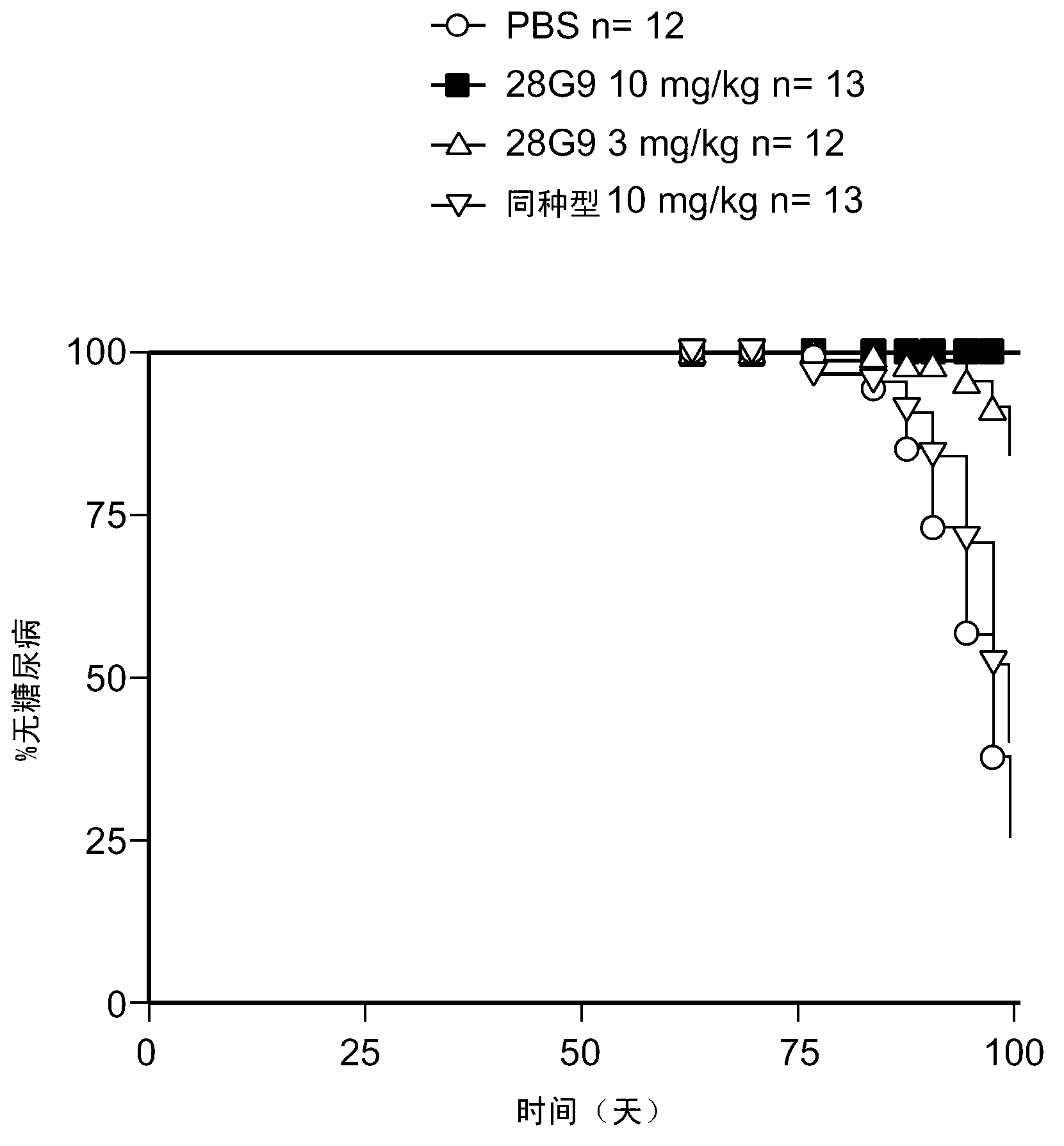
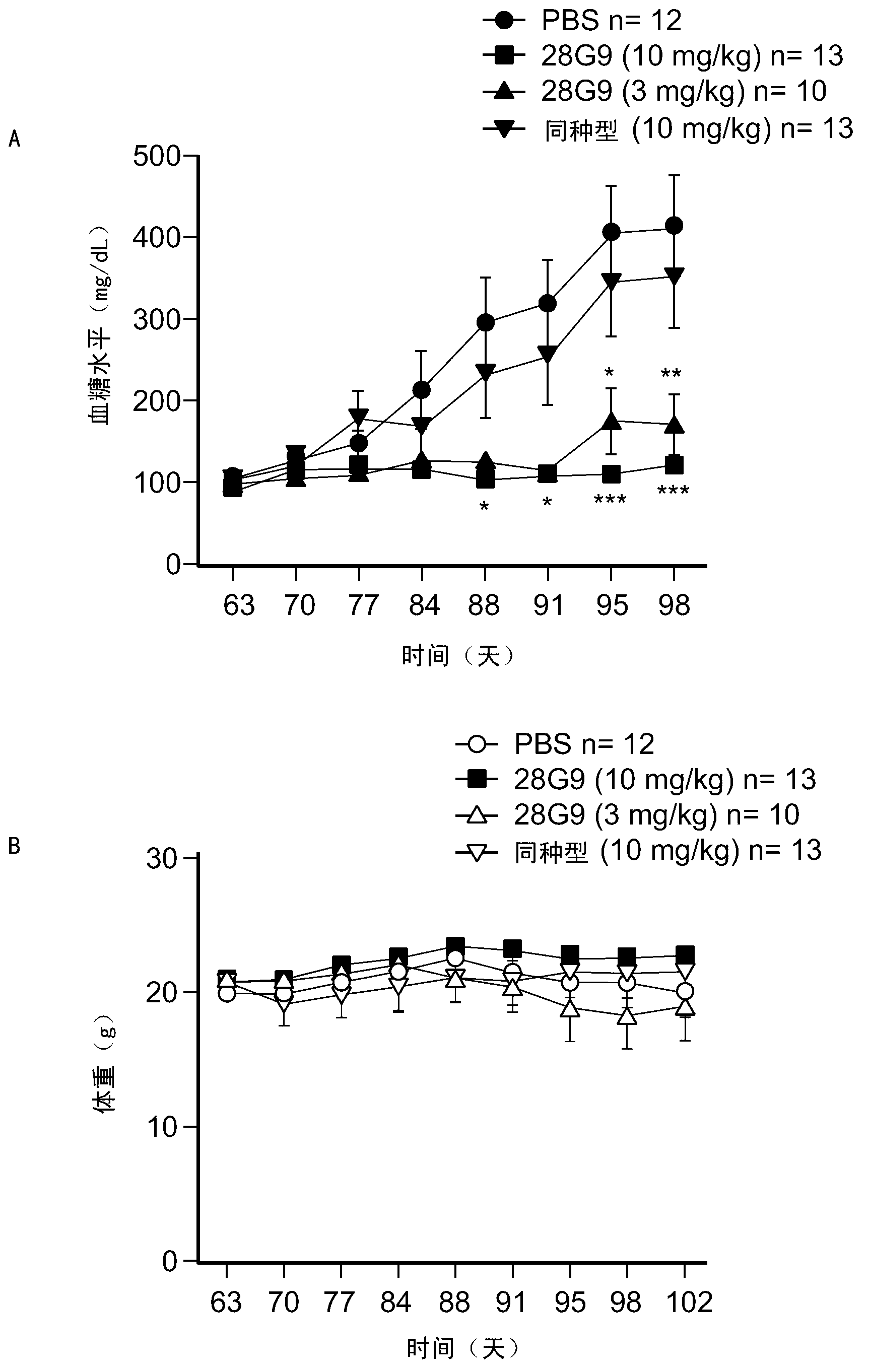
[0325] Example 4: Antagonistic IL-7R Antibodies Reduce Disease Incidence in Non-Obese Diabetic (NOD) Animals, Type 1 Diabetic Mouse Model
[0326]This example shows the effect of antagonist IL-7R antibodies in a mouse model of type 1 diabetes.
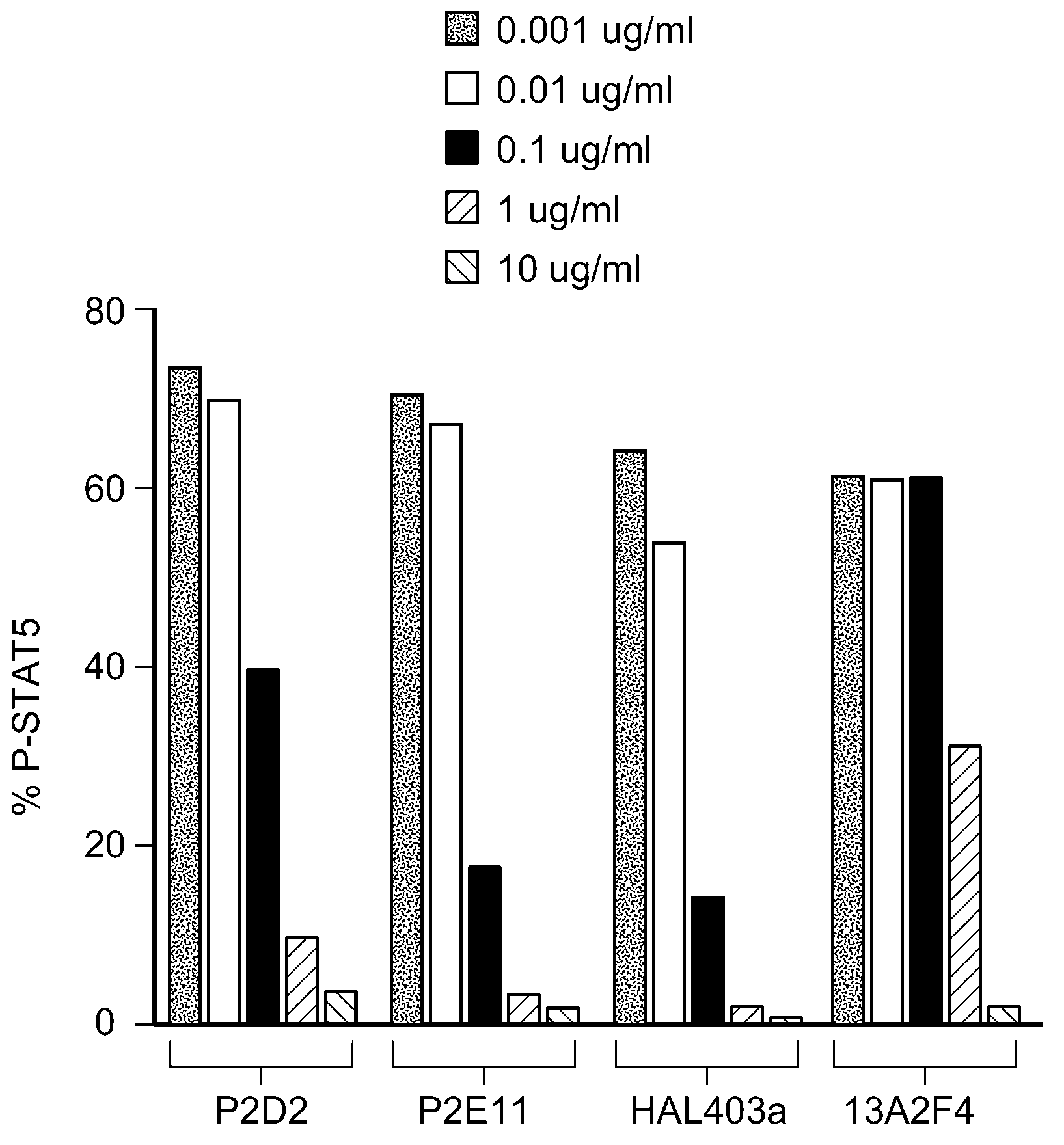
[0327] To study the in vivo effect of antagonist IL-7R antibodies on the diabetogenic process, the rat anti-mouse antagonist IL-7R antibody 28G9 (Rinat) was tested in NOD mice. NOD mice display a susceptibility to the spontaneous onset of autoimmune insulin-dependent diabetes mellitus (IDDM, type 1 diabetes) (Kikutani et al., 1992, Adv. Immunol. 51:285-322). 28G9 blocks IL-7-mediated STAT5 phosphorylation in mouse splenocytes and TM Medium cross competes with antagonist IL-7R human antibodies C1GM, C2M3, HAL403a, HAL403b, P3A9, P4B3, P2D2 and P2E11.
[0328] 6-8-week-old NOD female mice (The Jackson Laboratory) were injected intraperitoneally (i.p.) 3 or 10 mg / kg body weight of 28G9 weekly from 9 weeks of age (t=0). PBS or a non-r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com