Method for synthesizing cefoselis sulfate
A kind of technology of cefotaxime sulfate and synthesis method, applied in the direction of organic chemistry and the like, can solve the problems such as difficulty in purchasing cefa mother nucleus raw material, unfavorable continuity, large-scale industrial production, limited application prospect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
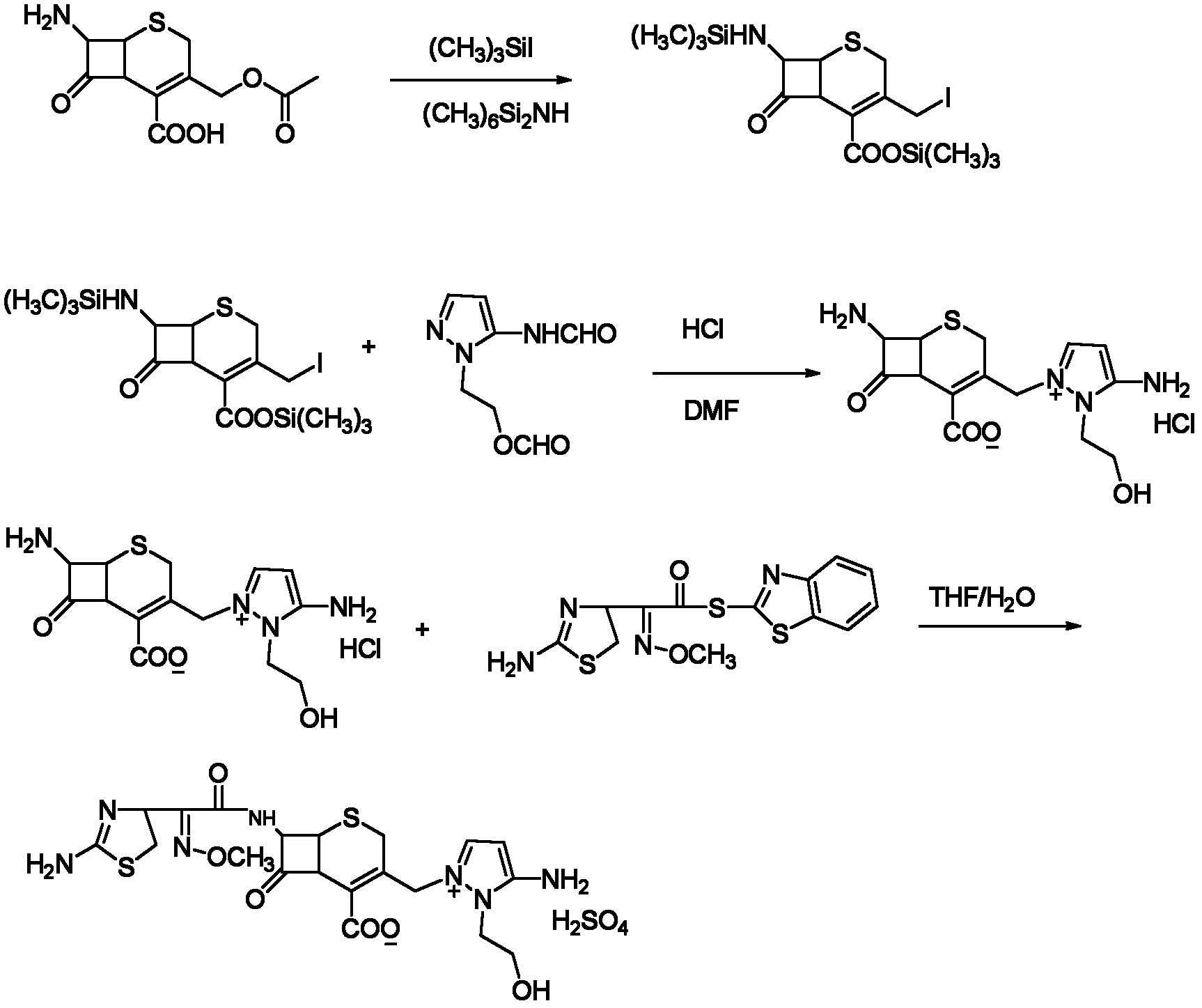
[0022] The synthetic method of embodiment 1 cefotaxime sulfate of the present invention
[0023]In the 2000ml four-neck flask equipped with stirrer, thermometer, nitrogen conduit and constant pressure dropping funnel, add 80g (0.295mol) 7-aminocephalosporanic acid (7-ACA), 800g dichloromethane, stir and cool to 10 ℃ or so. Add 110g (0.68mol) hexamethyldisilazane amine, 1g iodotrimethylsilane, 1g N,N-dimethylformamide under stirring, then naturally rise to room temperature, and stir and react at room temperature for 5-12 hours; Then the temperature was lowered to about -5°C, and under the protection of nitrogen, 125 g (0.625 mol) of iodotrimethylsilane was added dropwise. After the addition was complete, the reaction temperature was controlled at 0-5°C, and the reaction was stirred for 2 hours. Within this temperature range, 80 g (0.44 mol) of 5-formamidoamino-1-(2-formyloxyethyl)pyrazole was slowly added in portions. The reaction was stirred overnight at 0 °C. The next day,...
Embodiment 2
[0025] The synthetic method of embodiment 2 cefotaxime sulfate of the present invention
[0026] 7β-amino-3-[3-amino-2-(2-hydroxyethyl)-1-pyrazolamine methyl]-3-cephem-4-carboxylic acid hydrochloride synthesis: in 2000ml four Add 40g (0.147mol) of 7-aminocephalosporanic acid (7-ACA) and 800g of acetonitrile into the flask, stir and cool to about 10°C. Add 80g (0.5mol) hexamethyldisilazane amine, 1g iodotrimethylsilane, 1g N,N-dimethylformamide under stirring, then naturally rise to room temperature, and stir and react at room temperature for 5-12 hours; Then cool down to about -5°C, under the protection of nitrogen, add 80g (0.4mol) of iodotrimethylsilane dropwise, after the addition is completed, the reaction temperature is controlled at 0~-5°C, and the reaction is stirred for 2h; within this temperature range, Slowly add in batches, 80g (0.437mol) 5-formamidoamino-1-(2-formyloxyethyl)pyrazole; stir and react overnight at 0°C; slowly raise the temperature to 14°C the next da...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com