Use of a hydrophilic matrix comprising a polyacrylic acid derivative, a cellulose ether and a disintegrant for the manufacture of a medicament for treating female genital disorders
A technology of polyacrylic acid and cellulose ether, which is applied in diseases, medical preparations containing active ingredients, drug combinations, etc., can solve the problems of inability to achieve solid-gel transition, tablet retention, etc., and achieve improved chemical and physical properties. Effects of stability, pH reduction, and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
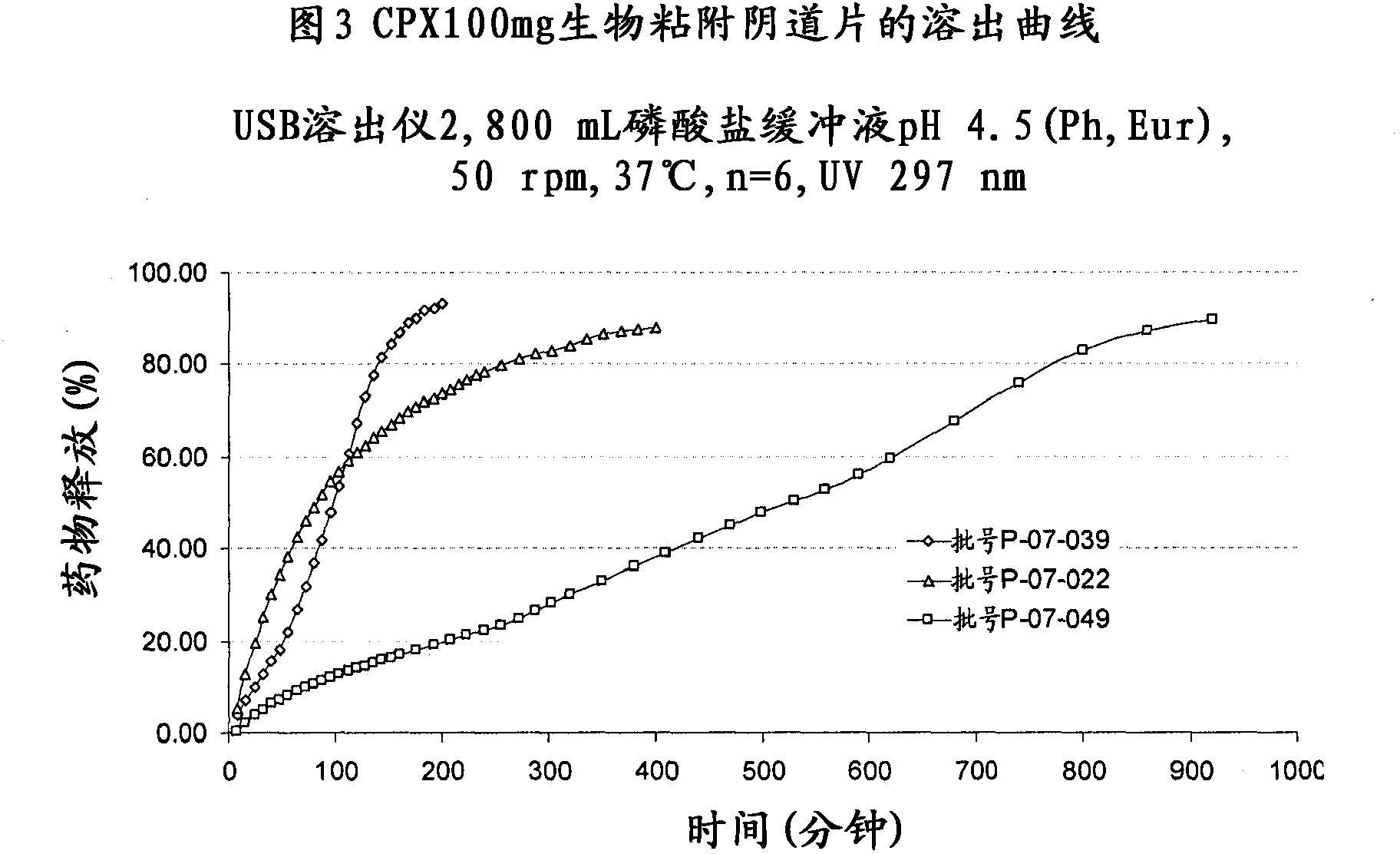
Examples
Embodiment 1
[0085] Embodiment 1 (comparative example)
[0086] Ciclopirox olamine 100 mg controlled-release bioadhesive vaginal tablets with lot number P06-037 were produced according to conditions known in the art.
[0087] Qualitative-quantitative formulations are shown in Table I.
[0088] Table I
[0089]
[0090] The method consisted of blending ingredients 1-8 followed by direct compression using a rotary tablet press (Officine Ronchi). A tablet weighing 760 mg with a hardness of 201N is obtained. Components No. 2 and No. 3 are components in the hydrophilic matrix (i).
Embodiment 2
[0092] Ciclopirox olamine 100 mg controlled release bioadhesive vaginal tablets with lot number P06-038 were produced according to the teachings of the present invention.
[0093] Tablets were prepared as described in Example 1.
[0094] Qualitative-quantitative formulations are shown in Table II.
[0095] Table II
[0096]
[0097] Components No. 2, No. 3 and No. 4 are components in the hydrophilic matrix (i).
[0098] A tablet weighing 760 mg with a hardness of 248N is obtained.
Embodiment 3
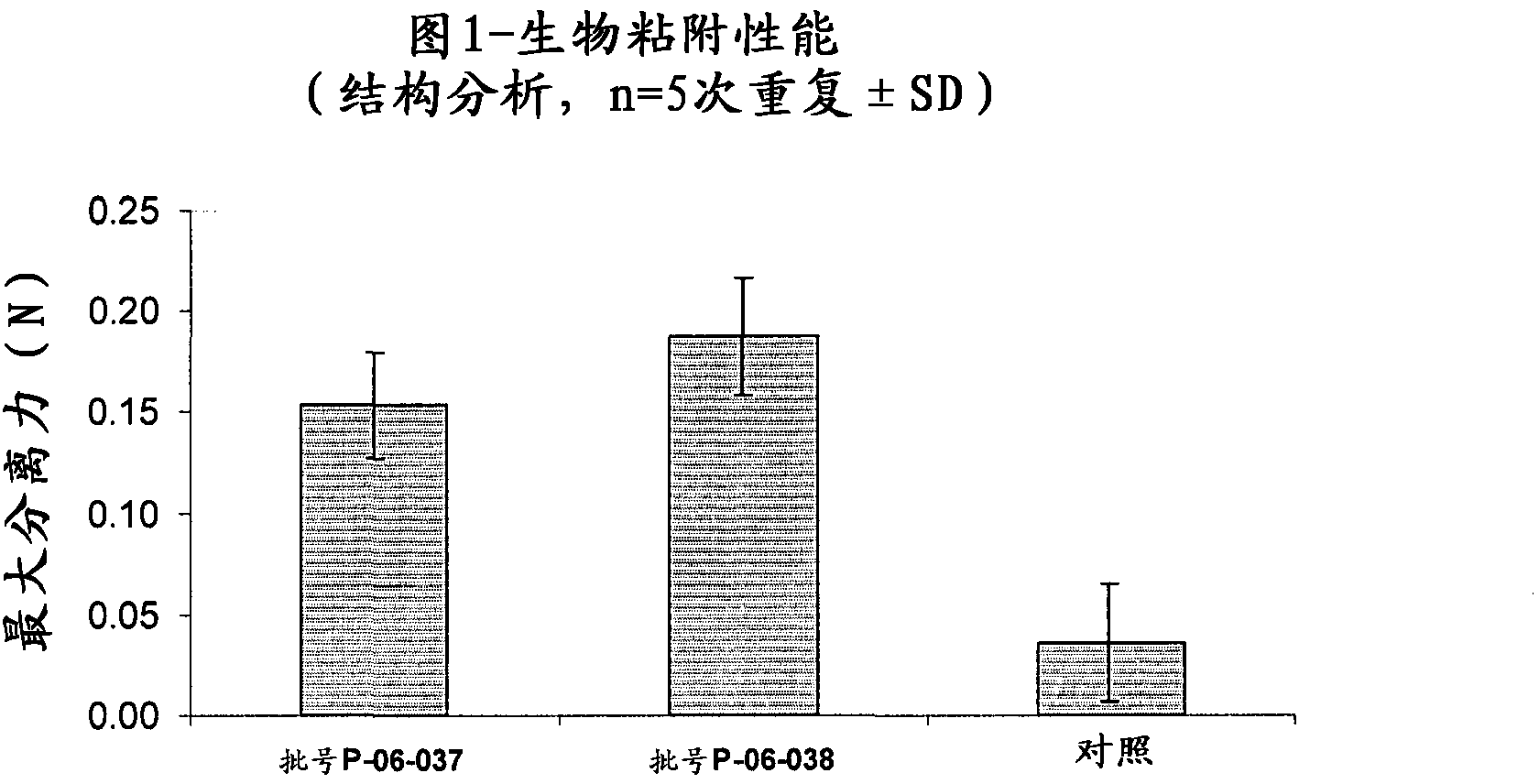
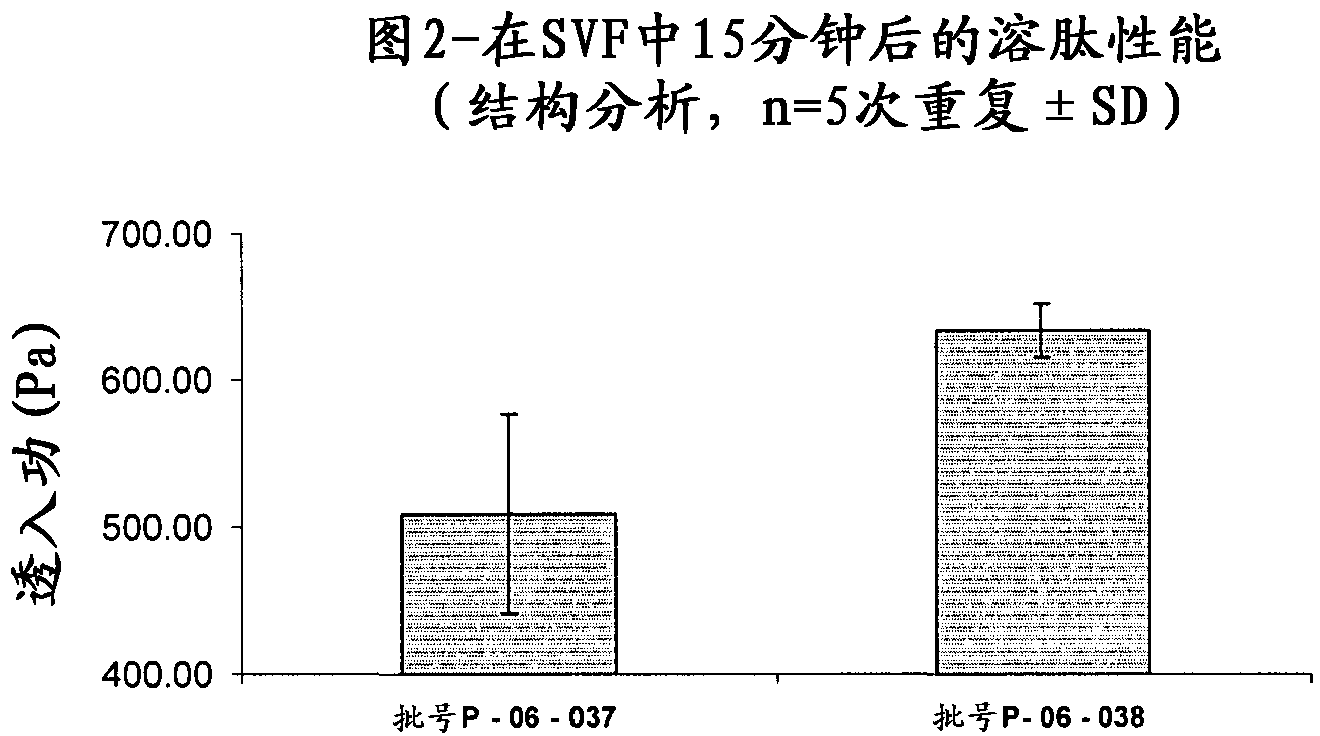
[0100] Structural analysis measurements were carried out on the tablets described in Example 1 (Lot No. P-06-037) and Example 2 (Lot No. P-06-038) in order to evaluate their bioadhesive properties and their degree of swelling. Tests for significant differences between means were performed by one-way ANOVA. Differences at the P<0.05 level were considered significant.
[0101] The bioadhesive properties were evaluated by a structure analyzer [1] and porcine vaginal mucosa was used as biosubstrate in order to simulate vaginal application.
[0102] A software-controlled dynamometer (AG / MC Acquati) with a 5 daN force cell was used to measure the separation force.
[0103] Sample Preparation
[0104] vaginal mucosa
[0105] The connective tissue was stripped from the vaginal mucosa obtained from the slaughterhouse using surgical scissors and stored at -20°C until use. The mucous membrane was thawed and carefully inspected visually for any defects prior to experimental applicati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com