Catalyst and application of catalyst in technology for preparing 1,5-pentanediol through hydrogenolysis of tetrahydrofurfuryl alcohol
A technology of tetrahydrofurfuryl alcohol and catalyst, which is applied in the direction of Raney catalyst, physical/chemical process catalyst, reduction preparation of oxygen-containing functional groups, etc., can solve the problems of no development prospect, complicated by-products, high production cost, etc., and achieve good industrialization The effect of application prospect, cheap raw materials and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
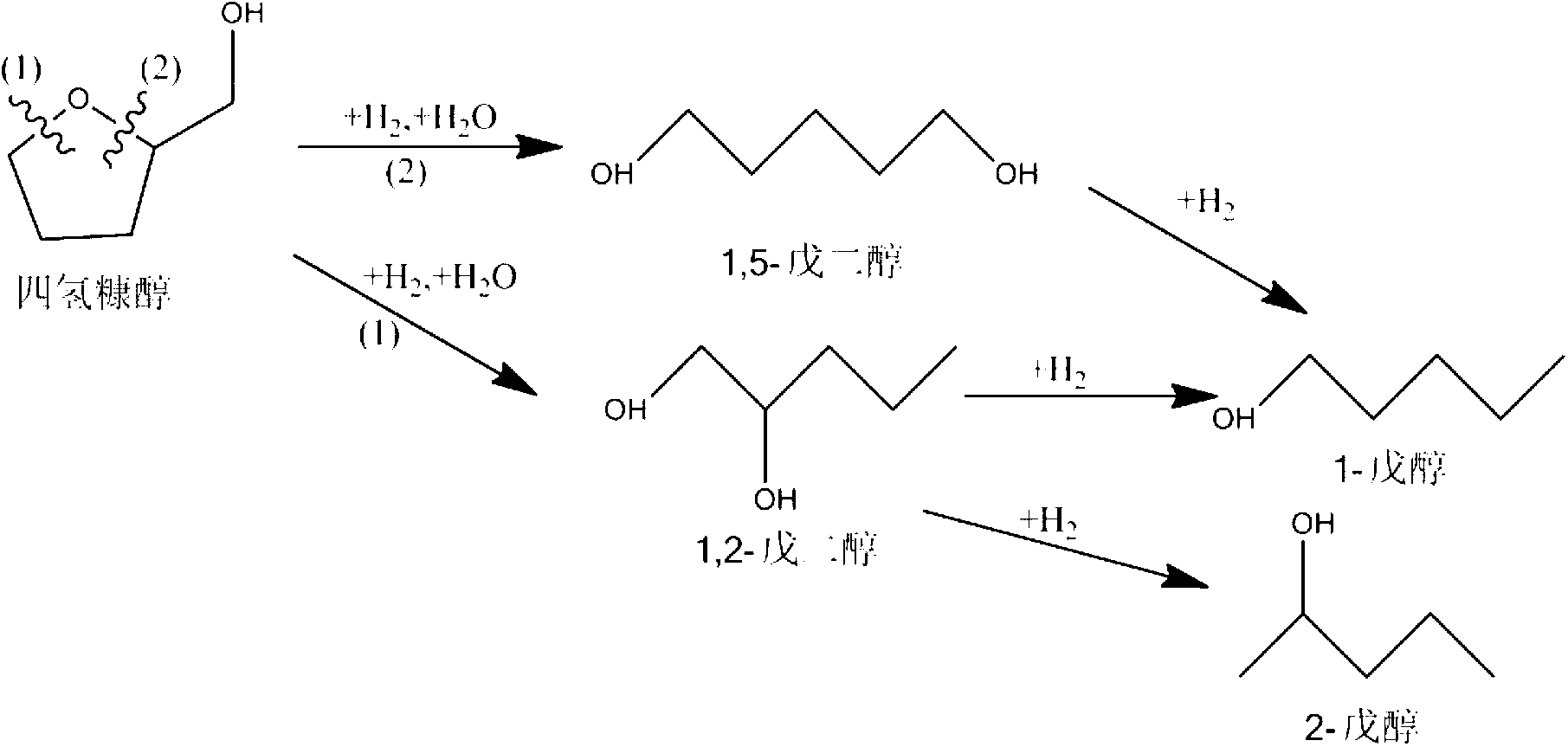
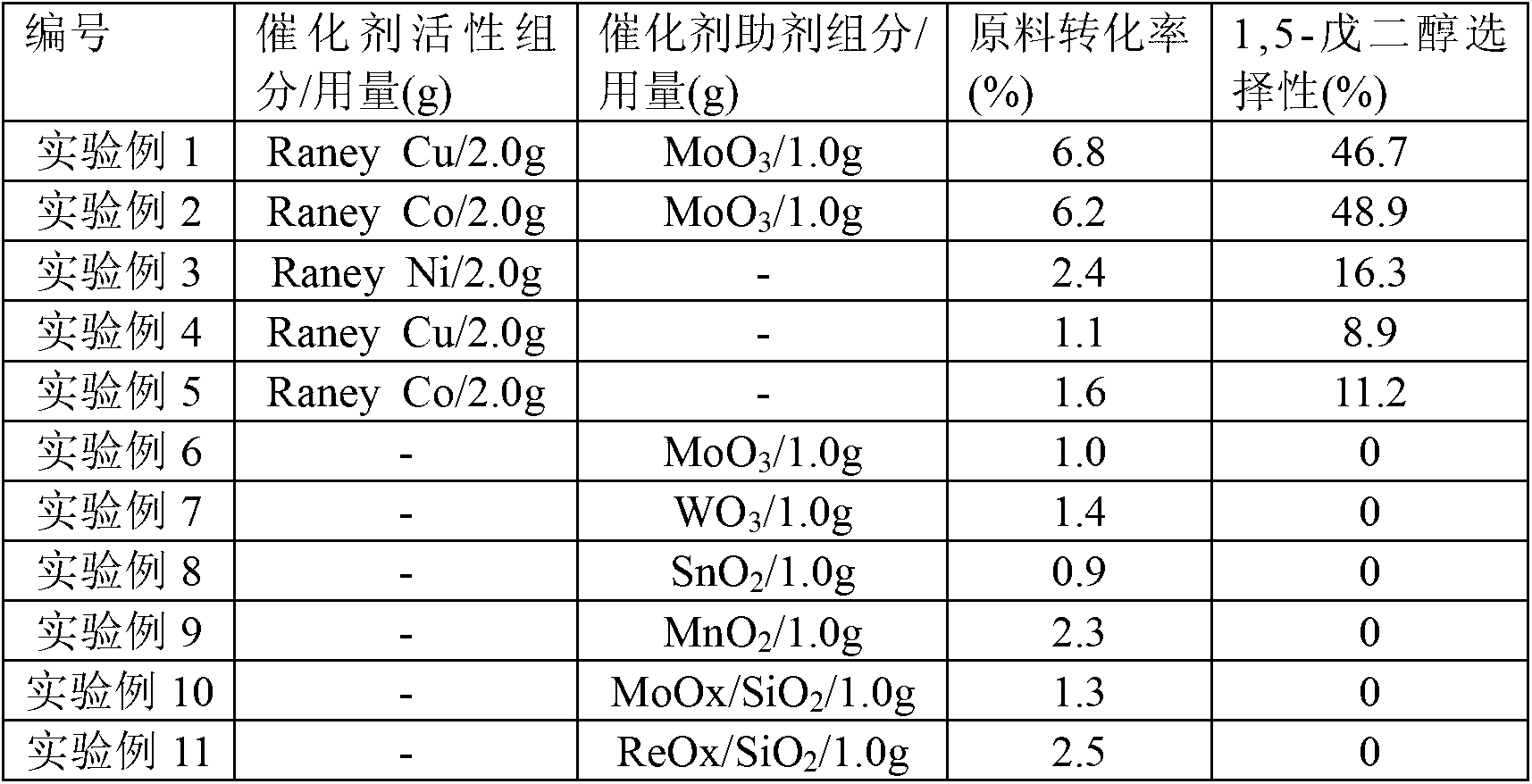
[0030] Get the Raney copper prepared in 2.0g synthesis embodiment 1 and 1.0g MoO 3 Put it into the high-pressure zirconium material reaction kettle, add 50mL of tetrahydrofurfuryl alcohol raw material solution containing 2% concentration, evacuate the reaction kettle with a vacuum pump, then flush 0.5MPa hydrogen gas, vent, and cycle three times. After cleaning the reaction kettle and pipeline, flush Inject hydrogen gas at a pressure of 8 MPa, stir at a speed of 300 rpm, and raise the temperature to 120° C. to start the reaction. After 24 hours of reaction, samples were taken for analysis and detection. The raw material conversion rate was 6.8%, and the selectivity to 1,5-pentanediol was 46.7%.
experiment example 2 to 11
[0032] 1,5-Pentanediol was prepared in the same process as in Experimental Example 1, except that the catalyst used in Table 1 below was used instead of the catalyst used in Experimental Example 1. The catalysts, raw material conversions and 1,5-pentanediol selectivities used in Experimental Examples 1 to 11 are listed in Table 1.
[0033] Table 1
[0034]
experiment example 12
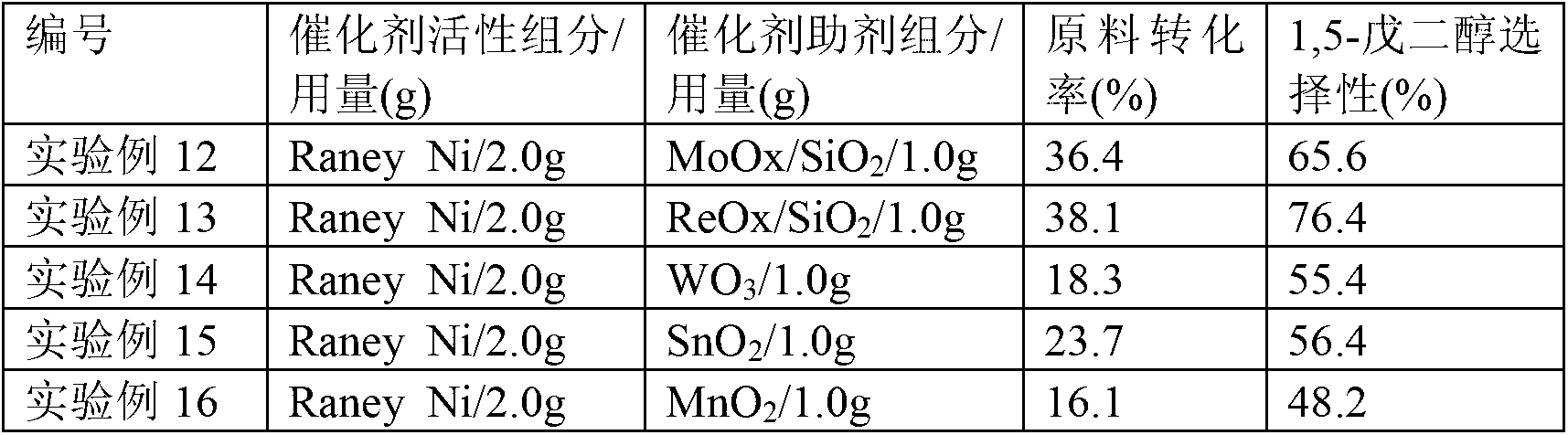
[0036] Get the MoOx / SiO prepared in 2.0g Synthetic Example 3 and the MoOx / SiO prepared in 1.0g Synthetic Example 4 2 Put it into the high-pressure zirconium material reaction kettle, add 50mL of tetrahydrofurfuryl alcohol raw material solution containing 2% concentration, evacuate the reaction kettle with a vacuum pump, then flush 0.5MPa hydrogen gas, vent, and cycle three times. After cleaning the reaction kettle and pipeline, flush Inject hydrogen gas at a pressure of 8 MPa, stir at a speed of 300 rpm, and raise the temperature to 180° C. to start the reaction. After 24 hours of reaction, samples were taken for analysis and detection. The raw material conversion rate was 36.4%, the selectivity to 1,5-pentanediol was 65.6%, the selectivity to n-pentanol was 24.7%, and the selectivity to 2-pentanol was 4.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com